Animals mostly have two sexes: males and females, and they produce offspring through sexual reproduction. As a consequence of evolution, animals show tremendous sexually dimorphic traits, which are often attractive and capture our attention. Many of these dimorphisms are regulated by genes belonging to the Doublesex and Mab-3 Related Transcriptional factor (DMRT) family. Despite this commonality, scientists have revealed the functional diversity of DMRT genes among animal taxa.
Sex-determining DMRT genes generally only contribute to male determination in vertebrates, nematodes, and arthropods other than insects. In contrast, a unique function can be seen in an insect DMRT gene,
doublesex, of holometabola insects other than bees and wasps. This function is the double-switching system, which is regulated by sexually unique splicing and is essential for both male and female morphogenesis. Since information on non-holometabolan insects has been fragmented and there has been a knowledge gap between insects and non-insect arthropods such as water fleas, it has been unclear how the
doublesex gene acquired its new function, that is, its essential role in female development.
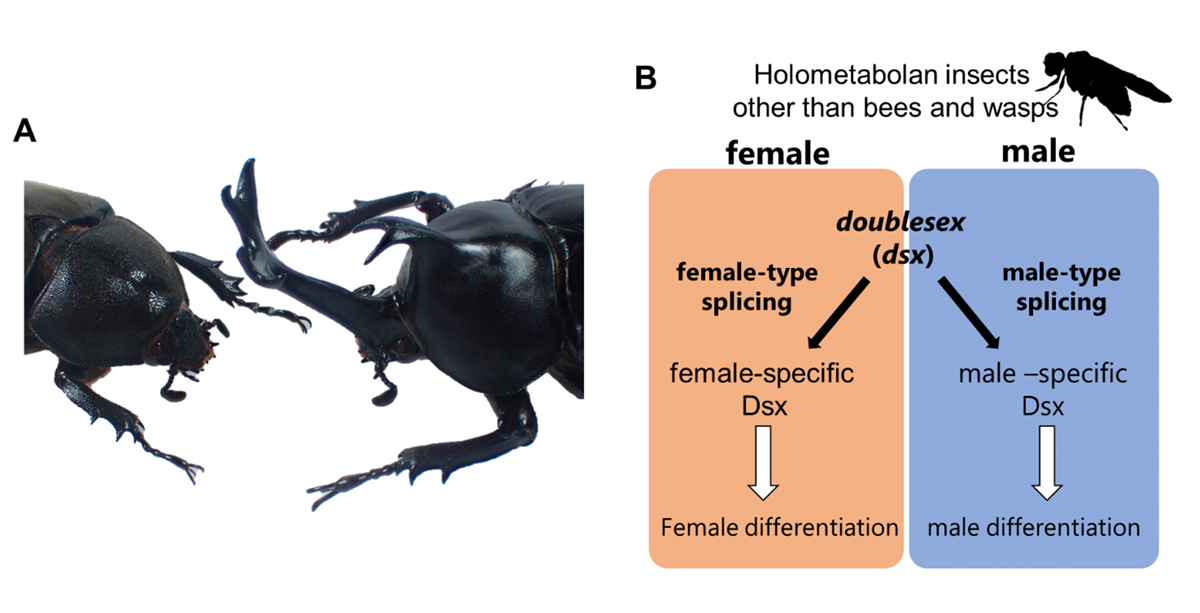 Figure 1: (A) Sexual dimorphism in insects. The rhinoceros beetle, Trypoxylus dichotomus, is shown as a representative species. (B) Molecular basis of sexual differentiation in insects. doublesex produces sex-specific isoforms and promotes both male and female differentiation in holometabolan insects other than bees and wasps.
Figure 1: (A) Sexual dimorphism in insects. The rhinoceros beetle, Trypoxylus dichotomus, is shown as a representative species. (B) Molecular basis of sexual differentiation in insects. doublesex produces sex-specific isoforms and promotes both male and female differentiation in holometabolan insects other than bees and wasps.
To infer the evolutionary history of the
doublesex gene, a research group led by the National Institute for Basic Biology (NIBB) revealed features of
doublesex in
Thermobia domestica, a species of Zygentoma that is the sister group of winged insects. Their findings were published in
Molecular Biology and Evolution. They proposed a novel hypothesis on the evolution of the double-switching system of insect sexual differentiation, in which
doublesex initially obtained a female-specific isoform controlling female-specific gene expression other than morphogenesis and later became essential for female morphogenesis via an extension of the C-terminus of the female-specific isoform.
"The double-switching system by
doublesex in insects is a significant phenomenon for investigating how a single gene acquires new roles opposite to existing functions as well as sexual differentiation of animals," stated Yasuhiko Chikami, the first author and a researcher of the Division of Evolutionary Developmental Biology, NIBB.
Initially, the researchers attempted to trace the evolutionary divergence of
doublesex through the
in silico analysis. They found that
doublesex was duplicated before the divergence of Zygentoma and winged insects. "The duplication event may have been overlooked since many winged insects lost one of two
doublesex copies," said Chikami. "Fortunately, species of Zygentoma conserved two paralogs of
doublesex, which suggests that Zygentoma may reflect the ancestral state of
doublesex in insects."
Scientists focused on the firebrat,
Thermobia domestica, a species of Zygentoma. This species has been maintained in their laboratory for more than a decade. "The firebrat is the key species to unveil evolutionary origins of many features in insects," said Prof. Teruyuki Niimi, who leads the Division of Evolutionary Developmental Biology at NIBB. "We have concentrated on the species for a long time and have previously developed technologies to analyze gene functions."
 Figure 2: Thermobia domestica. T. domestica is a species of Zygentoma. Zygentoma maintains many features found in the common ancestors of insects, such as non-winged nature and ametabolic growth.
Figure 2: Thermobia domestica. T. domestica is a species of Zygentoma. Zygentoma maintains many features found in the common ancestors of insects, such as non-winged nature and ametabolic growth.
They determined the gene structure of
doublesex in
T. domestica by RNA and genome sequencings and investigated its function in sexual morphology using the RNA interference method. The firebrat
doublesex has male- and female-specific isoforms and regulates male genital organs. However, it has no effect on female genital organs. These findings strongly support the hypothesis that the common ancestors of insects obtained sex-specific splicing control for
doublesex, and female differentiation subsequently became dependent on it. However, they also found a sex-antagonistic role of
doublesex in
T. domestica. "Unexpectedly,
doublesex upregulates
vitellogenin genes, which encode yolk protein precursors, in females and downregulates them in males," explained Chikami. "Our discovery suggests that the function of
doublesex for female morphogenesis emerged independently of roles for other biological processes in females."
A remaining question is how
doublesex obtained its roles in female morphogenesis. The researchers performed an ancestral sequence reconstruction of the female-specific Doublesex protein and AI-based protein structure prediction. They found that the C-terminal sequences of Doublesex are specific to the species whose
doublesex contributes to female morphogenesis. "The extension of the C-terminus may be associated with the neo-functionalization of
doublesex in insects," Chikami summarized.
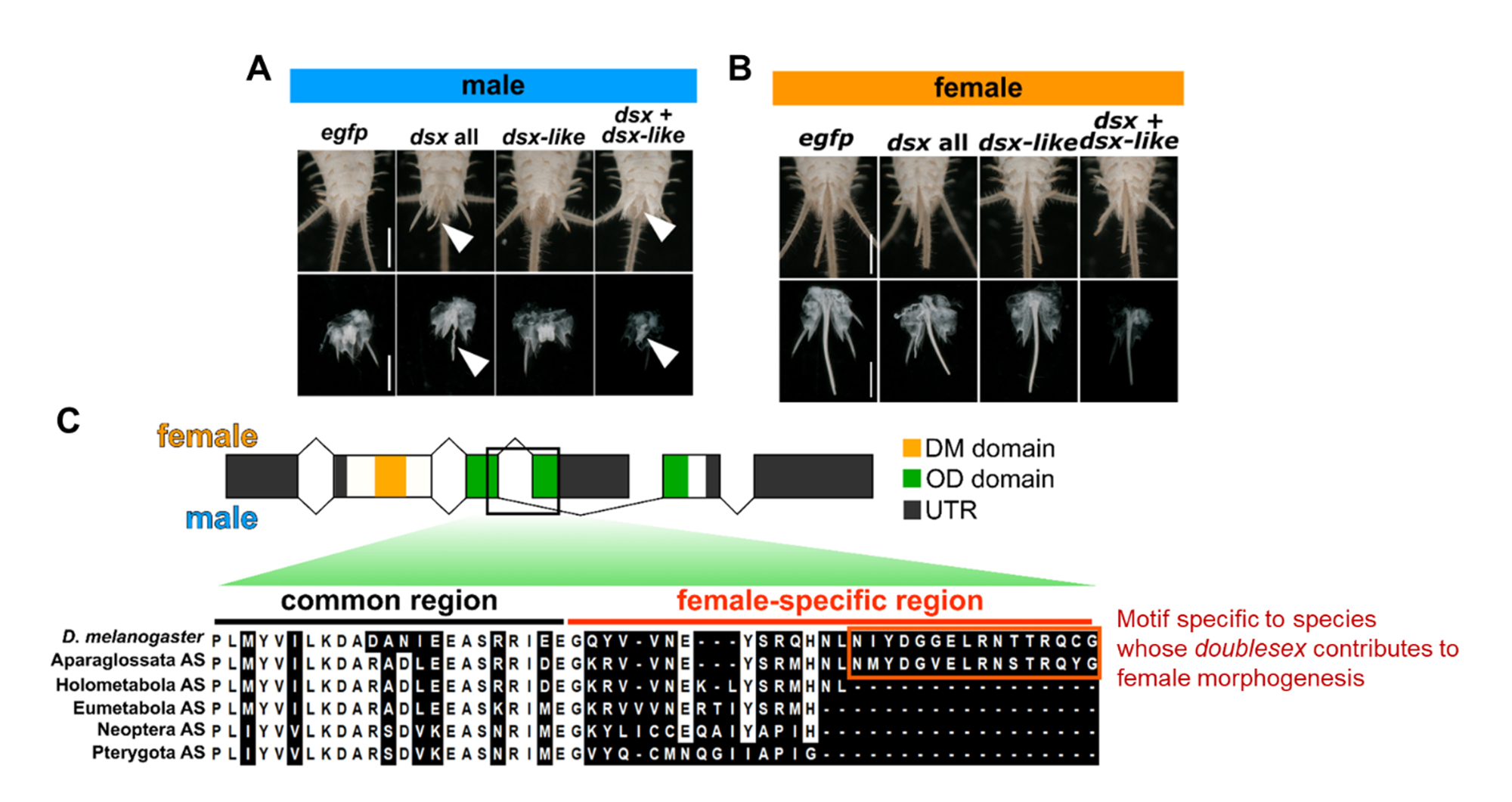 Figure 3: (A) The function of doublesex in males. Knockdown of doublesex leads to an ovipositor-like structure in males (white arrowheads). (B) The function of doublesex in females. We could not detect any effect on female traits due to the knockdown of doublesex. (C) Evolution of the C-terminal sequences of the female-specific Doublesex protein. Species with doublesex, which is essential for female morphogenesis, have a specific motif at the C-terminus of Doublesex (orange frame).
Figure 3: (A) The function of doublesex in males. Knockdown of doublesex leads to an ovipositor-like structure in males (white arrowheads). (B) The function of doublesex in females. We could not detect any effect on female traits due to the knockdown of doublesex. (C) Evolution of the C-terminal sequences of the female-specific Doublesex protein. Species with doublesex, which is essential for female morphogenesis, have a specific motif at the C-terminus of Doublesex (orange frame).
Prof. Niimi commented, "Our results update the conventional hypothesis on the evolutionary origin of the double-switching system in insect sexual differentiation." "There are many examples of neo-functionalization of genes by coding mutations," said Chikami. "Furthermore, our findings suggest that a single gene acquires alternative isoforms and later, through altering protein motifs of an isoform, may procure a new function that is opposite to its existing roles," he added at the end.
Article Information:
Molecular Biology and Evolution
Authors: Yasuhiko Chikami, Miki Okuno, Atsushi Toyoda, Takehiko Itoh, Teruyuki Niimi
Title: Evolutionary history of sexual differentiation mechanism in insects
DOI:
10.1093/molbev/msac145
Contact:
Prof. Teruyuki Niimi
Division of Evolutionary Developmental Biology
National Institute for Basic Biology
E-mail: niimi@nibb.ac.jp




 Figure 1: (A) Sexual dimorphism in insects. The rhinoceros beetle, Trypoxylus dichotomus, is shown as a representative species. (B) Molecular basis of sexual differentiation in insects. doublesex produces sex-specific isoforms and promotes both male and female differentiation in holometabolan insects other than bees and wasps.
Figure 1: (A) Sexual dimorphism in insects. The rhinoceros beetle, Trypoxylus dichotomus, is shown as a representative species. (B) Molecular basis of sexual differentiation in insects. doublesex produces sex-specific isoforms and promotes both male and female differentiation in holometabolan insects other than bees and wasps. Figure 2: Thermobia domestica. T. domestica is a species of Zygentoma. Zygentoma maintains many features found in the common ancestors of insects, such as non-winged nature and ametabolic growth.
Figure 2: Thermobia domestica. T. domestica is a species of Zygentoma. Zygentoma maintains many features found in the common ancestors of insects, such as non-winged nature and ametabolic growth. Figure 3: (A) The function of doublesex in males. Knockdown of doublesex leads to an ovipositor-like structure in males (white arrowheads). (B) The function of doublesex in females. We could not detect any effect on female traits due to the knockdown of doublesex. (C) Evolution of the C-terminal sequences of the female-specific Doublesex protein. Species with doublesex, which is essential for female morphogenesis, have a specific motif at the C-terminus of Doublesex (orange frame).
Figure 3: (A) The function of doublesex in males. Knockdown of doublesex leads to an ovipositor-like structure in males (white arrowheads). (B) The function of doublesex in females. We could not detect any effect on female traits due to the knockdown of doublesex. (C) Evolution of the C-terminal sequences of the female-specific Doublesex protein. Species with doublesex, which is essential for female morphogenesis, have a specific motif at the C-terminus of Doublesex (orange frame).