Just like in yeast and animal cells, vacuoles in plants are responsible for breaking down unwanted cellular components. At the same time, vacuoles in seeds also serve the opposite role -storing large amounts of proteins that provide energy during germination. These storage proteins, accumulated in the vacuoles of seeds such as beans and wheat, are not only vital for plant growth but also represent an important agricultural resource closely tied to our daily diet.
Until now, it was entirely unknown whether proteins could be transported
from the vacuole to other organelles. In a study now published in
Nature Plants, Dr. Yihong Feng (Specially Appointed Assistant Professor) and Professor Takashi Ueda at the National Institute for Basic Biology in Japan, together with their collaborators, demonstrated in the model plant
Arabidopsis thaliana the existence of a retrograde trafficking pathway that retrieves the membrane protein VAMP727 from the vacuolar membrane back to endosomes. The team further identified the molecular machinery responsible for this pathway.
Dr. Feng commented, “The sorting nexin proteins that function in this newly discovered pathway have independently diversified in plants compared to animals and yeast. Our findings indicate that this trafficking route is a plant-specific innovation.”
Professor Ueda added, “We were able to show that the plant-unique membrane fusion protein VAMP727 co-evolved with a recycling mechanism from the vacuole. This suggests that the evolution of vacuolar protein transport in seed plants, which is essential for massive storage protein accumulation, was closely tied to the emergence of this retrograde pathway.”
This study reveals part of the unique membrane trafficking network that plants have evolved during their history, providing new insights into both cell biology and plant science.
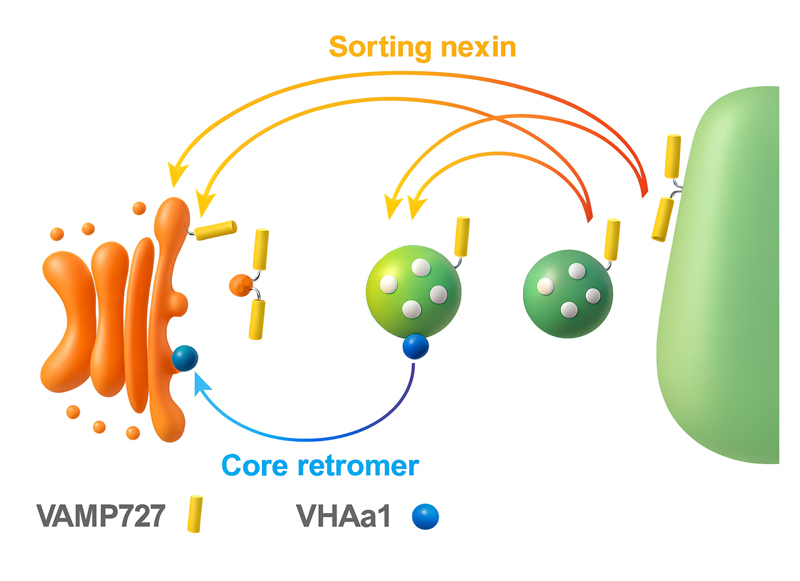 Figure 1. Retrograde trafficking pathways from the plant vacuole and endosomes.
Figure 1. Retrograde trafficking pathways from the plant vacuole and endosomes.
This study revealed two independent retrograde pathways: a sorting nexin–mediated pathway (orange) and a core retromer–mediated pathway (blue). In yeast, sorting nexins and the core retromer form a single complex that functions in endosome-to-Golgi retrograde transport. In contrast, our findings demonstrate that in plants, SNX and the core retromer operate independently, mediating distinct retrograde routes with different cargo proteins. Together, these pathways establish and maintain the unique membrane protein compositions of vacuoles and endosomes.
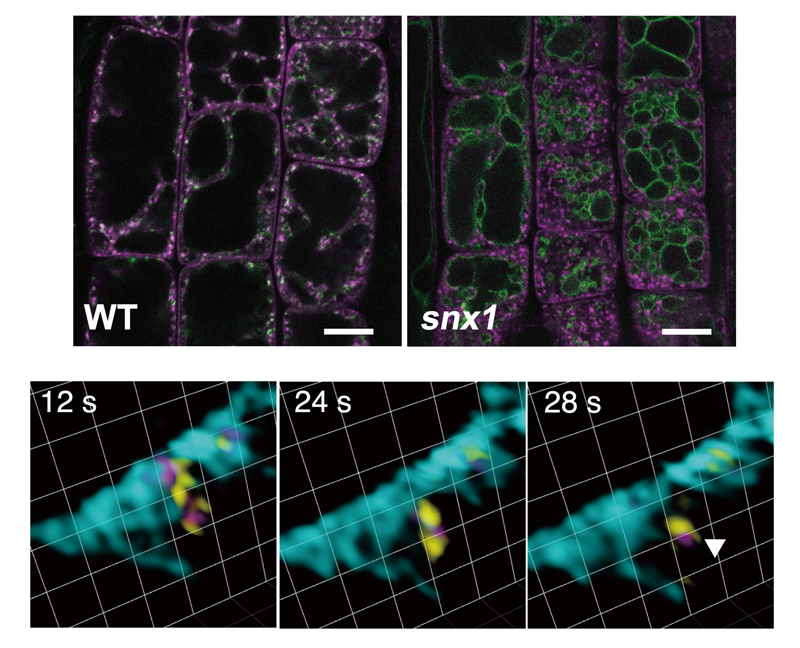 Figure 2. VAMP727 is retrieved from the vacuolar membrane by SORTING NEXINs.
Top panels:
Figure 2. VAMP727 is retrieved from the vacuolar membrane by SORTING NEXINs.
Top panels: Root epidermal cells expressing Venus-VAMP727 and mRFP-VAMP721 in wild type (left) and the
snx1 mutant (right). The punctate localization pattern of VAMP721, which functions in the secretory pathway, remained unchanged in the
snx1 mutant. In contrast, VAMP727 accumulated at the vacuolar membrane in the
snx1 background. Scale bar = 10 µm.
Bottom panels: Images acquired using the super-resolution confocal live imaging microscope (SCLIM) of mGFP-VAMP727 (yellow), SNX1-mRFP (magenta), and the vacuolar membrane marker miRFP-SYP22 (cyan). These images capture the budding of VAMP727 together with SNX1 from the vacuolar membrane. Grid size = 0.942 µm.
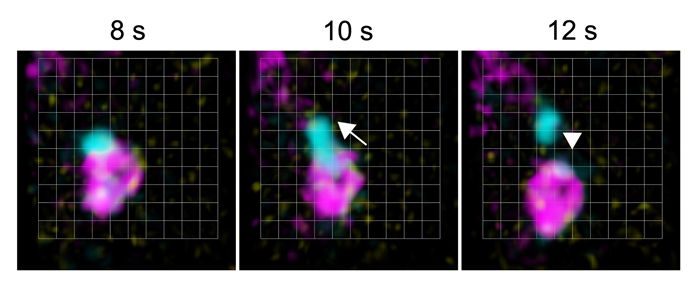 Figure 3. SNX and the core retromer function in distinct retrograde pathways.
Figure 3. SNX and the core retromer function in distinct retrograde pathways.
Images acquired using the SCLIM of VPS35b-iRFP (core retromer, cyan), SNX1-mRFP (magenta), and the endosomal marker GFP-ARA7 (yellow). Time-lapse observations revealed budding of VPS35b from the endosomal membrane, which did not include SNX1. Grid size = 0.426 µm.
Nature Plants
Retrieval from vacuolar/endosomal compartments underpinning neofunctionalization of SNARE in plants
Yihong Feng, Kazuo Ebine, Yoko Ito, Takehiko Kanazawa, Tatsuya Sawasaki, Akira Nozawa, Tomohiro Uemura, Akihiko Nakano, Takashi Ueda
DOI:
10.1038/s41477-025-02115-5




 Figure 1. Retrograde trafficking pathways from the plant vacuole and endosomes.
Figure 1. Retrograde trafficking pathways from the plant vacuole and endosomes. Figure 2. VAMP727 is retrieved from the vacuolar membrane by SORTING NEXINs.
Figure 2. VAMP727 is retrieved from the vacuolar membrane by SORTING NEXINs. Figure 3. SNX and the core retromer function in distinct retrograde pathways.
Figure 3. SNX and the core retromer function in distinct retrograde pathways.