
National Institute for Basic Biology




The Optics and Imaging Facility assists both collaborative and core research by managing and maintaining research tools that use Light. The facility, under the guidance of Dr. Kamei, also provides technical support through the management of technical staff assisting in the advancement of collaborative and core research projects, as well as academic support to researchers (please refer to the Collaborative Research Group Research Enhancement Strategy Office section for more information). Among the equipment available are advanced biological microscopes, and the Okazaki Large Spectrograph for photobiology. The Okazaki Large Spectrograph is the world's largest wide spectrum exposure mechanism, and is capable of producing a range of wavelengths from 250 nm (ultraviolet) to 1,000 nm (infrared) along its 10-meter focal curve, thus allowing exposure to strong monochromatic light. The facility's microscopes, which include cutting edge devices such as confocal and multi-photon excitation microscopes, are an indispensable part of core and collaborative projects conducted by both internal and external researchers.
The spectrograph runs on a 30 kW Xenon arc lamp and projects a wavelength spectrum ranging from 250 nm (ultraviolet) to 1,000 nm (infrared) onto its 10 m focal curve with an intensity of monochromatic light at each wavelength more than twice as great as that of the corresponding monochromatic component of tropical sunlight at noon (Watanabe et al., Photochem. Photobiol. 36, 491-498, 1982). The spectrograph is dedicated to action spectroscopical studies of various light-controlled biological processes.
In addition to the other action spectroscopical studies concerning various regulatory and damaging effects of light on living organisms, research involving both biological and artificial organic molecules have been conducted since it has been set up. The NIBB Collaborative Research Program for the Use of the OLS supports about 10 projects every year conducted by both visiting scientists, including foreign researchers, as well as members of NIBB.
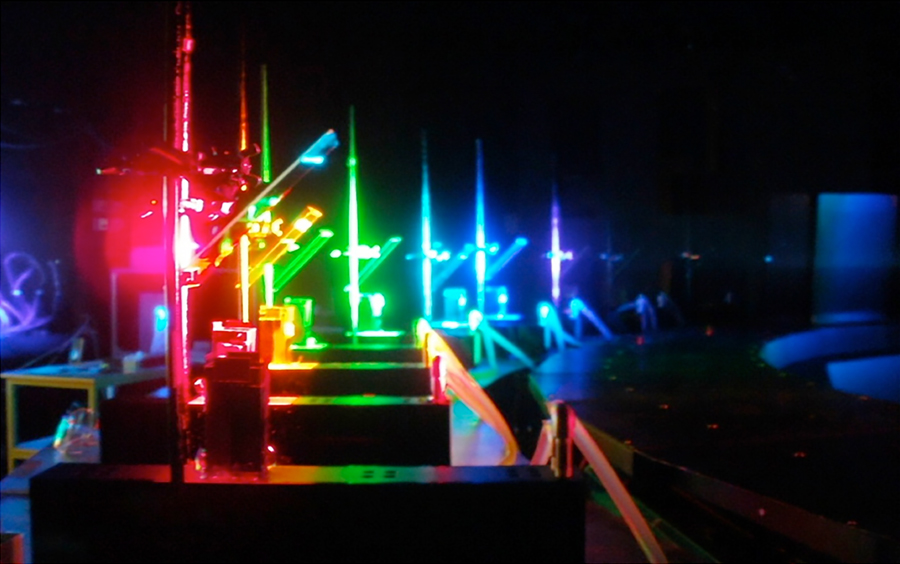
Figure 1. An example of an experiment using the Large Spectrograph. In this photo, various color rays (monochromatic light from right side and reflected by mirrors) are irradiated simultaneously onto samples stored in cooling chambers.
This facility also provides bioimaging machinery (Figure 2), such as wide-field microscopes (Olympus IX-81 and BX-63), confocal microscopes (Leica TCS-SP8, Nikon A1R, Nikon A1Rsi and Yokogawa CSU-X1 with EM-CCD/ CMOS cameras), multi-photon microscopes (Olympus FV1000-MP, FV1200-MPs, Leica TCS-SP8 MPs) and other advanced laser microscopes boasting specialized, cutting edge technology (Light-sheet Microscope and Infrared Laser-Evoked Gene Operator microscope: IR-LEGO), which can be utilized by researchers within NIBB, as well as collaborative guest researchers. Starting from 2016, we have commenced two new types of Collaborative Research Programs. One is a new category within the NIBB Collaborative Research for Integrative Bioimaging program using machinery and bioimage processing/analysis techniques, and the other is the Advanced Bioimaging Support Program (ABiS) which operates under the framework of the Grant-in-Aid for Scientific Research on Innovative Areas.
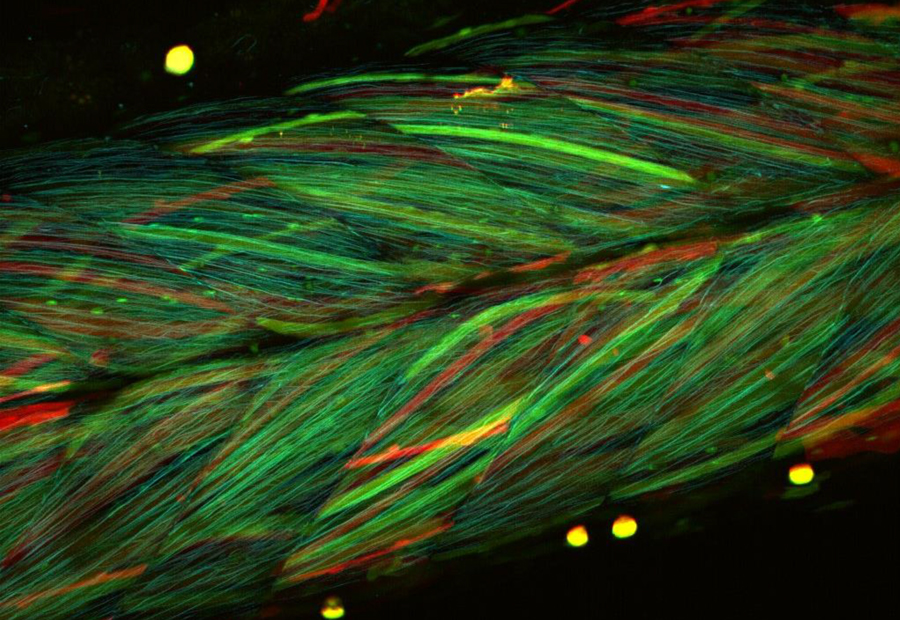
Figure 2. Multi-color confocal microscope image of muscle in a transgenic medaka larva. Each muscle cell expressed randomly various color variants of fluorescent protein, such as Cerulean, GFP, YFP and dsRed. The transgenic line was provided by NBRP Medaka.
The light-sheet microscope was developed by Dr. Ernst Stelzer’s group at the European Molecular Biology Laboratory (EMBL). This microscope can realize high-speed z-axis scanning in deeper tissues by illuminating specimens from the side with a light sheet (more information is given in the report submitted by Dr. Shigenori Nonaka’s Laboratory for Spatiotemporal Regulations). Subsequently, Dr. Nonaka has conducted and supported roughly 10 Collaborative Research Program projects for Integrative Bioimaging. The IR-LEGO, developed by Drs. Shunsuke Yuba and Yasuhiro Kamei at the National Institute of Advanced Industrial Science and Technology (AIST), can induce a target gene of interest by heating a single target cell in vivo with a high efficiency irradiating infrared laser. This microscope technology can be applied to thermal biology through local heating (the details of this are provided in the next section). IR-LEGO was also used for about 10 Collaborative Research projects, including applications aimed at both animals and plants.
In 2021, we held the 9th biological image processing training course in cooperation with Drs. Kagayaki Kato, Shigenori Nonaka, Yasuhiro Kamei, Takashi Murata and Hiroshi Koyama. The course was held in an online meeting format due to the COVID-19 pandemic. We have also started a new course “Optical Microscopy Principle Training Course” cooperation with Drs. Joe Sakamoto, Yasuhiro Kamei, Atsushi Taniguchi, Shigenori Nonaka in NIBB and Motosuke Tsutsumi and Kohei Otomo in ExCELLS. In this course, participants learned microscopy principles thorough lectures and built bright-field and fluorescent microscopes by themselves using optical devices, such as lenses, filters, light sources and cameras (Figure 3). We additionally hold some training courses and seminars related to microscopy and image analysis, including their technologies and applications.
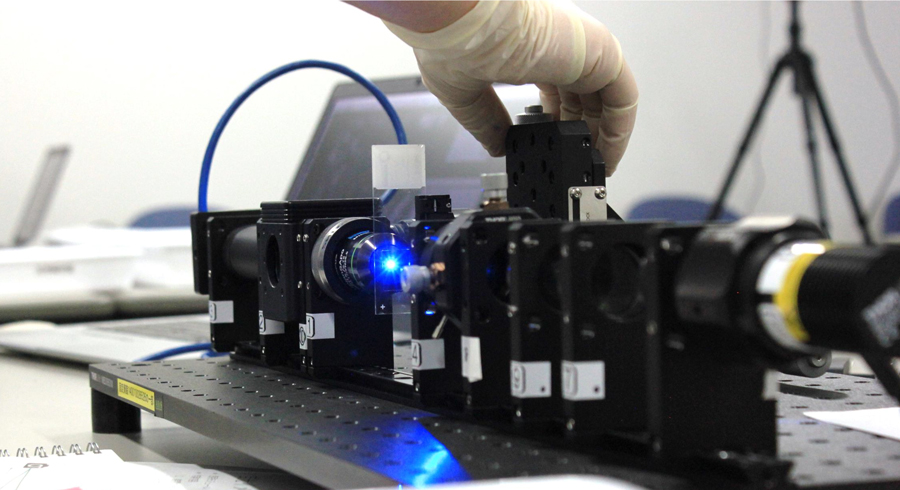
Figure 3. A scene from the 1st Optical Microscopy Principle Training Course. The participants built up a basic microscope by themselves using optical devices.
RMC Professor KAMEI, Yasuhiro TEL: +81 564 55 7535
E-mail : ykamei@nibb.ac.jp
