
National Institute for Basic Biology





(Written in 2018)
We have been studying the molecular and cellular mechanisms underlying the development of the vertebrate central nervous system. The scope of our interests also encompasses mechanisms for various functions of the mature brain, including sensation, emotion, behavior, learning and memory. The main research themes are as follows.
Please see http://niwww3.nibb.ac.jp/ for details.
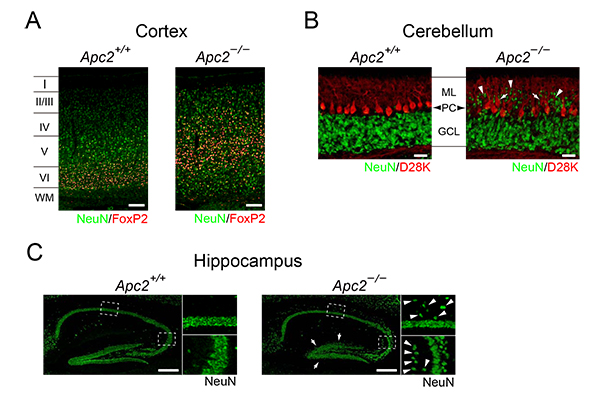
Figure 1. Disorganized lamination in the Apc2-deficient brain
A. In the wild-type cortex, FoxP2-positive cells are distributed in the layer IV. In contrast, in the Apc2-deficient cortex, they are broadly distributed in the upper layers.
B. In the mutant cerebellum, granule cells (indicated by arrowheads) are abnormally distributed in the molecular layer (ML), and the layering of Purkinje cells (arrows) was also affected.
C. In the mutant hippocampus, many pyramidal cells are ectopically distributed (arrowheads). In addition, dentate granule cells are less densely packed in the mutant (arrows).
The APC2 deficiency was revealed to be a crucial cause for Sotos syndrome (see Almuriekhi et al., Cell Reports 10, 1585-1598, 2015).
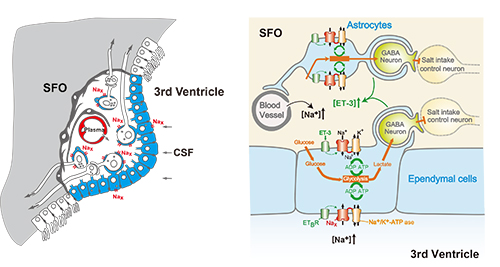
Figure 2. Overview of the [Na+]-sensing mechanism and Nax-dependent regulation of neuronal activity in the SFO
For details, see Noda and Sakuta, Trends Neurosci. 36, 661-673, 2013.
Matsuda T., Hiyama TY, Niimuara F, Matsusaka T, Fukamizu A, Kobayashi K, Kobayashi K and Noda M. Distinct neural mechanisms for the control of thirst ans salt appetite in the subfornical organ. Nature Neurosci. 20, 230-241, 2017.
Almuriekhi, M., Shintani, T., Fahiminiya, S., Fujikawa, A., Kuboyama, K., Takeuchi, Y., Nawaz, Z., Nadaf, J., Kamel, H., Kitam, A.K., Samiha, Z., Mahmoud, L., Ben-Omran, T., Majewski, J., and Noda, M. (2015). Loss-of-function mutation in APC2 causes Sotos Syndrome features. Cell Reports 10, 1585-1598.
Hiyama, T.Y., Yoshida, M., Matsumoto, M., Suzuki, R., Matsuda, T., Watanabe, E., and Noda, M. (2013). Endothelin-3 expression in the subfornical organ enhances the sensitivity of Nax, the brain sodium-level sensor, to suppress salt intake. Cell Metab. 17, 507-519.
Hiyama, T.Y., Matsuda, S., Fujikawa, A., Matsumoto, M., Watanabe, E., Kajiwara, H., Niimura, F., and Noda, M. (2010). Autoimmunity to the sodium-level sensor in the brain causes essential hypernatremia. Neuron 66, 508-522.
Shimizu, H., Watanabe, E., Hiyama, T.Y., Nagakura, A., Fujikawa, A., Okado, H., Yanagawa, Y., Obata, K., and Noda, M. (2007). Glial Nax channels control lactate signaling to neurons for brain [Na+] sensing. Neuron 54, 59-72.
Shintani, T., Ihara, M., Sakuta, H., Takahashi, H., Watakabe, I., and Noda, M. (2006). Eph receptors are negatively controlled by protein tyrosine phosphatase receptor type O. Nature Neurosci. 9, 761-769.
Fujikawa, A., Shirasaka, D., Yamamoto, S., Ota, H., Yahiro, K., Fukada, M., Shintani, T., Wada, A., Aoyama, N., Hirayama, T., Fukamachi, H., and Noda, M. (2003). Mice deficient in protein tyrosine phosphatase receptor type Z are resistant to gastric ulcer induction by VacA of Helicobacter pylori. Nature Gen. 33, 375-381.