
National Institute for Basic Biology





Since plants spread their roots in the ground, they must survive in a given environment. To adapt to the environment, they positively utilize environmental changes in the life cycle as important signals that are necessary for their survival (Figure 1A). Plant cells can induce, degenerate and differentiate their organelles to adapt to environmental changes. The flexibility of plant organelles is the basis of the strategy for environmental adaptation in plants.
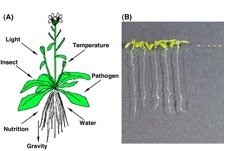
Figure 1: Various stimuli from environment and peroxisome function on germination. (A) Plants recognize environmental stimuli and utilize them as signals for survival. (B) Wild-type plants (left), but not ped1 mutants (right), can germinate on medium without sucrose. ped1 mutants are defective in ß-oxidation of peroxisomes so that they are not able to utilize storage lipids for gluconeogenesis.
The aim of this division is to clarify the molecular mechanisms underlying induction, differentiation, and interaction of organelles, and to understand the integrated function of individual plants (Figure 1B) through organelle dynamics, especially peroxisomes (Figure 2B and 2C), vacuoles (Figure 2A, 2B and 2D) and ER-derived vesicles (Figure 2E).
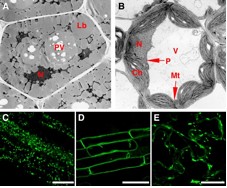 Figure 2: Functional transition of organelles in cotyledonary cells and visualization of organelles. The morphology, number and function of organelles in plant cells are different among the types of tissues, organs and developmental stage. Shown are electron micrographs of cotyledonary cells of seeds (A) and green tissues (B). N: nucleus, PV: protein storage vacuole, Lb: lipid body, V: vacuole, P: peroxisome, Ch: chloroplast, Mt: mitochondrion. Peroxisomes (C) and vacuolar membrane (D) in the root, and endoplasmic reticulum (ER) in the cotyledon (E) are visualized with green fluorescent protein (GFP). Rod-shape structures in (E) are ER bodies. Bars indicate 50 µm.
Figure 2: Functional transition of organelles in cotyledonary cells and visualization of organelles. The morphology, number and function of organelles in plant cells are different among the types of tissues, organs and developmental stage. Shown are electron micrographs of cotyledonary cells of seeds (A) and green tissues (B). N: nucleus, PV: protein storage vacuole, Lb: lipid body, V: vacuole, P: peroxisome, Ch: chloroplast, Mt: mitochondrion. Peroxisomes (C) and vacuolar membrane (D) in the root, and endoplasmic reticulum (ER) in the cotyledon (E) are visualized with green fluorescent protein (GFP). Rod-shape structures in (E) are ER bodies. Bars indicate 50 µm.
The function of plant organelles is different among tissues, organs and developmental stages (Figure 2A and 2B). Glyoxysomes, which are peroxisomes engaged in the degradation of reserve oil via β-oxidation and the glyoxylate cycle, are transformed into leaf peroxisomes that function in photorespiration. After the functional transition of glyoxysomes to leaf peroxisomes during the greening of pumpkin cotyledons, the reverse transition of leaf peroxisomes to glyoxysomes occurs during senescence. Gene expression, alternative splicing, protein translocation and protein degradation control the functional transformation between glyoxysomes and leaf peroxisomes. This organelle transformation supports the integrated function of plants.
Almost all of the peroxisomal matrix proteins are known to contain the targeting signal to peroxisomes (PTS) within the molecules. We identified 256 gene candidates of PTS-containing proteins and another 30 genes of non-PTS-containing proteins from Arabidopsis genome. Custom-made DNA microarray covering all these genes was used to investigate expression profiles of the peroxisomal genes in various organs. In parallel, we made a two-dimensional protein map of glyoxysomes and leaf peroxisomes isolated from Arabidopsis. Peptide MS fingerprinting analyses allowed us to identify novel proteins exists in peroxisomes.
Bioinfomatic analysis of Arabidopsis genome predicted the presence of 15 kinds of genes for peroxisomal biogenesis factors, called PEX genes. We comprehensively investigated whether these predicted PEX genes function in peroxisome biogenesis by generating knock-down mutants that suppress PEX gene expression by RNA-interference.
To better understand peroxisome biogenesis, we isolated a number of Arabidopsis mutants having aberrant peroxisome morphology (apm mutants) based on the different pattern of GFP fluorescence from the parent plant, GFP-PTS1, in which peroxisomes with normal size and number can be visualized with GFP (Figure 2C). Analyses of these apm mutants and identification of APM genes will identify components necessary for peroxisome biogenesis and address the regulation of its mechanism.
Plant cells develop various types of endoplasmic reticulum (ER)-derived structures with specific functions. ER bodies, which are surrounded by ribosomes, are rod-shaped structures (5 μm long and 0.5 μm wide), and are widely observed in the epidermal cells of whole seedlings (Figure 2E). Interestingly, rosette leaves of adult plants had no ER bodies, but ER bodies are accumulated after wounding or jasmonic acid treatment in this organ, suggesting that ER bodies have a function in the defense against herbivores. Arabidopsis nai1 mutant was isolated as a mutant that does not contain ER bodies. Transient expression of NAI1 induced ER bodies in the nai1 mutant, providing direct evidence that NAI1 plays a role in the formation of ER bodies. We are trying to isolate additional components that are involved in ER body formation.
The vacuolar processing enzyme (VPE) belongs to the cysteine protease family found in higher plants and animals. Plant VPE homologues are separated into three subfamilies: seed type, vegetative type and embryogenic type. Seed type VPE is responsible for the maturation of seed storage proteins. In contrast, the function of vegetative and embryogenic type VPEs was obscure. Recently, we revealed a novel function of VPE in various types of programmed cell death (PCD) in plants. The plant hypersensitive response (HR) constitutes well-organized PCD. No HR occurs on the tobacco mosaic virus-infected leaves of VPE-silenced tobacco plants (Figure 3A). Fumonisin B1 (FB1), a fungal toxin, induces cell death in Arabidopsis. The features of FB1-induced cell death were completely abolished in the Arabidopsis VPE-null mutant (Figure 3B). δVPE, an embryogenic type VPE, specifically and transiently expresses in two cell layers (ii2-ii3) of the seed coat at an early stage of seed development. At this stage, PCD accompanying cell shrinkage occurs in ii2-ii3. In a dvpe mutant, shrinkage of these layers was delayed (Figure 3C). An ultrastructural analysis showed that the disintegration of the vacuolar membranes occurs before the cell death in these PCDs. These results suggest that VPE is involved in PCD by vacuolar collapse, and that plants evolve a death strategy mediated by a vacuolar system, which is not seen in animals.
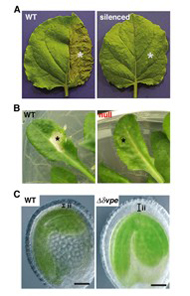
Figure 3: VPE deficiency suppresses various types of programmed cell death (PCD) in plants. (A) The non-silenced (WT) and VPE-silenced (silenced) Nicotiana benthamiana plants were infected with tobacco mosaic virus on halves of their leaves (indicated by asterisks). The photographs were taken after 24 hours. (B) Leaves of wild-type (WT) and VPE-null mutant (null) Arabidopsis plants were infiltrated with FB1, a fungal toxin (indicated by asterisks). The photographs were taken after 5 days. C, Thickness of inner integuments (ii) of the seed coats is reduced in wild-type (WT) seed at the early stage, whereas it is not reduced in the dvpe mutant seed of Arabidopsis. PCD accompanies the shrinkage of two cell layers of the seed coat in wild type seeds.
Molecular chaperones are cellular proteins that function in the folding and assembly of certain other polypeptides into oligomeric structures but that are not, themselves, components of the final oligomeric structure. To clarify the roles of molecular chaperones on cell differentiation, we have purified and characterized chaperonin and Hsp70s and analyzed their roles in the translocation of proteins into chloroplasts. Recently, we started to characterize HSP90s using a specific inhibitor of HSP90 and transgenic plants expressing mutated Arabidopsis HSP90.
The Plant Organelles Database 2 (PODB2) was built to promote a comprehensive understanding of organelle dynamics. This public database is open to all researchers. PODB2 consists of 4 individual parts: the organelles movie database, the organellome database, the functional analysis database, and external links. The organelles movie database contains videos of organelle movements and 3D structures (Figure 4). The organellome database provides images of various plant organelles that were visualized with fluorescent and nonfluorescent probes in various tissues of several plant species at different developmental stages. The functional analysis database is a collection of protocols for plant organelle research. The amount of included data is increasing day by day. It is expected that PODB2 will contribute to systems biology through the combination of the included data with other 'omics' data and computational analyses. In addition, we will release an updated version for educational use soon. We expect that PODB2 will be a useful tool to help researchers gain greater knowledge of plant organelles, as well as the general public who want to explore plant cell biology.
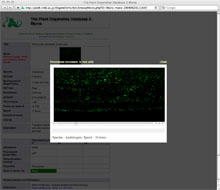
Figure 4: The graphical user interfaces of 'Organelles Movie Database' in PODB2 (http://podb.nibb.ac.jp/Orgenellome).
Hayashi, M., Nito, K., Toriyama-Kato, K., Kondo, M., Yamaya, T., and Nishimura, M. (2000). AtPex14 maintains peroxisomal functions by determining protein targeting to three kinds of plant peroxisomes. EMBO J. 19, 5701-5710.
Mano, S., Miwa, T., Nishikawa, S., Mimura, T., and Nishimura, M. (2008). The plant organelles database (PODB): A collection of visualized plant organelles and protocols for plant organelle research. Nucleic Acid Res. 36, D929-937.
Yamada, K., Nagano, A. J., Nishina, M., Hara-Nishimura, I., and Nishimura, M. (2008). NAI2 is an endoplasmic reticulum body component that enables ER body formation in Arabidopsis thaliana. Plant Cell 20, 2529-2540.
Arai, Y., Hayashi, M., and Nishimura, M. (2008). Proteomic identification and characterization of a novel peroxisomal adenine nucleotide transporter supplying ATP for fatty acid β-oxidation. Plant Cell 20, 3227-3240.
Goto, S., Mano, S., Nakamori, C., and Nishimura, M. (2011). Arabidopsis ABERRANT PEROXISOME MORPHOLOGY 9 is a peroxin that recruits the PEX1-PEX6 complex to peroxisomes. Plant Cell 23, 1573-1587.