
National Institute for Basic Biology




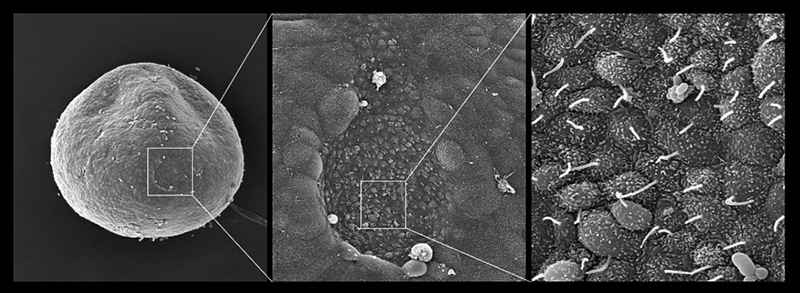
In mammalian development, the initial left-right asymmetry first appears as cilia-driven leftward fluid flow on the ventral surface of the node, and this flow provides the initial cue for asymmetric gene expressions that actually determine asymmetric morphogenesis. The mechanisms that convert the flow to asymmetric gene expression, i.e. the flow sensing mechanism, remain controversial, with several models being proposed, and the involvement of Ca2+ being suggested.
We pursued this question by measuring Ca2+ dynamics in the node and found that the node cells apparently cause stochastic elevation of Ca2+. The spatiotemporal distribution is equal on the left and right sides but becomes more prevalent on the left after the late headfold stage, when flow-induced commitment occurs. This asymmetry was disrupted in dynein mutant iv/iv and pkd2-/- mutants, in accordance to their left-right phenotypes.
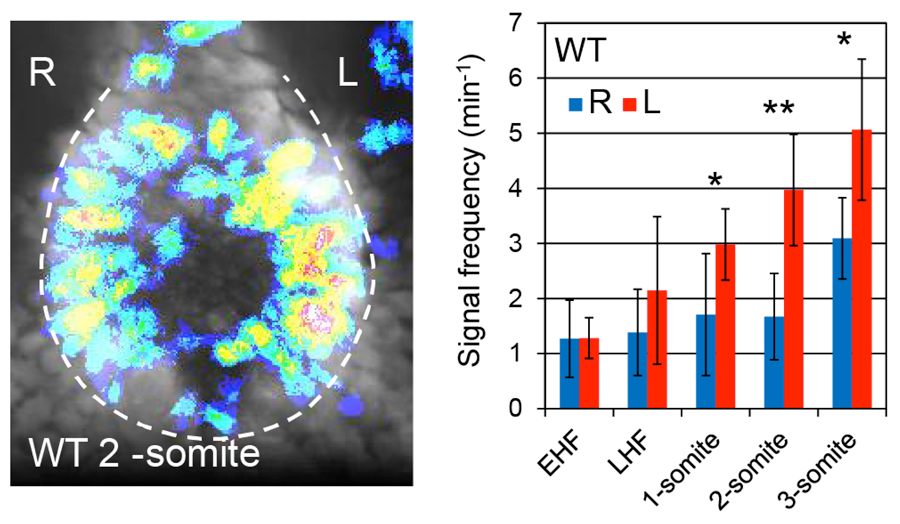
Figure 1. Left: Distribution of Ca2+ elevation in a 2-somite wild-type node. Right: Time course of Ca2+ elevation frequency at the left and the right sides.
Based on these findings, we are now examining the validity of the two major proposed models of flow sensing, mechanosensing and chemosensing, as well as a third novel mechanism.
Light-sheet microscopy has become popular during this decade for benefits such as low photodamage and fast image acquisition. We are now running two light-sheet microscopes, one commercial and the other self-made, and are maintaining them for collaborations and our own research interest (this being left-right asymmetry).
Over several years, we have developed a fast light-sheet microscope named ezDSLM, in which the excitation light sheet and detection objective move instead of the specimen for z-scanning. We installed an electrically tunable lens (ETL) to achieve greater speed and the exclusion of mechanical vibrations that disturb continuous 4D imaging of floating or loosely held specimens.
Our light-sheet microscopes are available to other researchers via NIBB’s Collaborative Research and MEXT’s Advanced Bioimaging Support (ABiS) programs. Numerous collaborations are in progress including observation of moving Amoeba proteus, neuronal activity in Drosophila larvae, cell migration in zebrafish embryos, cleared mouse brains, and marine crustaceans, etc.
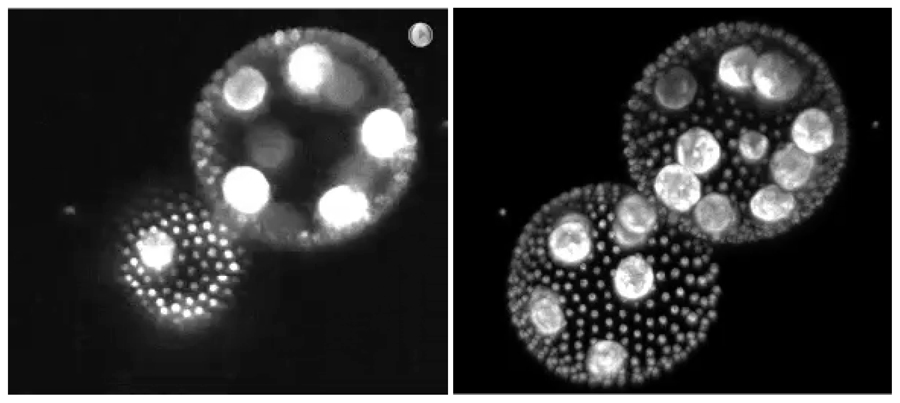
Figure 2. Images of floating volvox taken by ezDSLM with ETL. Left: Single optical section. Right: Maximum intensity projection.
This laboratory is currently recruiting graduate students.
Associate Professor NONAKA, Shigenori TEL: +81 564 55 7590 E-mail: snonaka@nibb.ac.jp
