Because plants spread their roots in the ground, they must survive in a given environment. To adapt to their environment, they utilized various signals generated from environmental changes as being necessary for their survival. As such, the flexibility of plant organelles is the basis for such adaptation. The aims of this laboratory are to clarify the molecular mechanisms underlying the induction, differentiation, and interaction of organelles, especially peroxisomes and oil bodies, as well as to understand the integrated functions of individual plants through organelle dynamics.
Research Projects
-
• Revealing the molecular mechanisms of peroxisome dynamics and functions in plant cells
-
• Analysis of the accumulation mechanisms of seed storage oils and proteins
-
• Development of Gateway-technology vectors for plant research
I. Molecular mechanisms of peroxisome dynamics and functions in plant cells
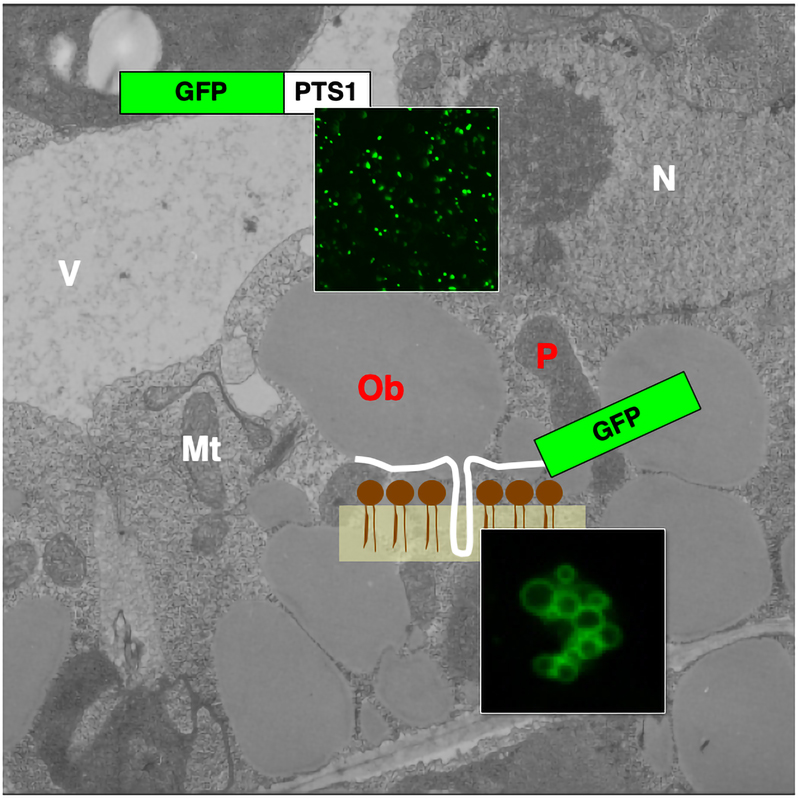
Peroxisomes are single-membrane-bound organelles, which are frequently present in eukaryotic cells, and are involved in various biological processes such as lipid metabolism and photorespiration. These functions change dramatically during certain developmental stages, and when they are confronted with environmental changes. For example, light induces the transformation of peroxisomes from glyoxysomes, which are peroxisomes engaged in the degradation of reserve oil stored in the oil body via b-oxidation and the glyoxylate cycle, to another type of peroxisome, leaf peroxisomes, that function during several crucial steps of photorespiration. In addition to functioning in vegetative tissues such as leaf and root cells, it has been revealed that peroxisomes play essential roles in reproductive processes. Studies using Arabidopsis mutants defective in peroxisomal functions demonstrate that peroxisomes contribute to pollen fertility, pollen tube elongation, and male–female gametophyte recognition. Gene expression, alternative splicing, protein transport, protein degradation and degradation of peroxisomes themselves control these functions.
To better understand peroxisome biogenesis and functions, we isolated a number of Arabidopsis mutants that displayed aberrant peroxisome morphology (
apem mutants) and unusual peroxisome positioning (
peup mutants) based on them having a different pattern of GFP fluorescence compared to their parent plant, GFP-PTS1, in which peroxisomes with normal sizes, numbers and distribution could be visualized with GFP (Figure 1). As of writing, we have reported the functions of APEM1, APEM2, APEM3, APEM4, APEM9 and APEM10. Based on these results we were able to update the model for protein transport, proliferation and quality control of peroxisomes via autophagy, using these
apem mutants in concert with the analyses of
peup1,
peup2,
peup4,
peup17 and
peup22 mutants, which were defective in
Autophagy-related (
ATG) genes.
We are also currently investigating other
apem and
peup mutants. Through these analyses, we will be able to identify the components responsible for peroxisome biogenesis, functions and maintenance, and address the mechanism underlying this phenomenon at the molecular level.
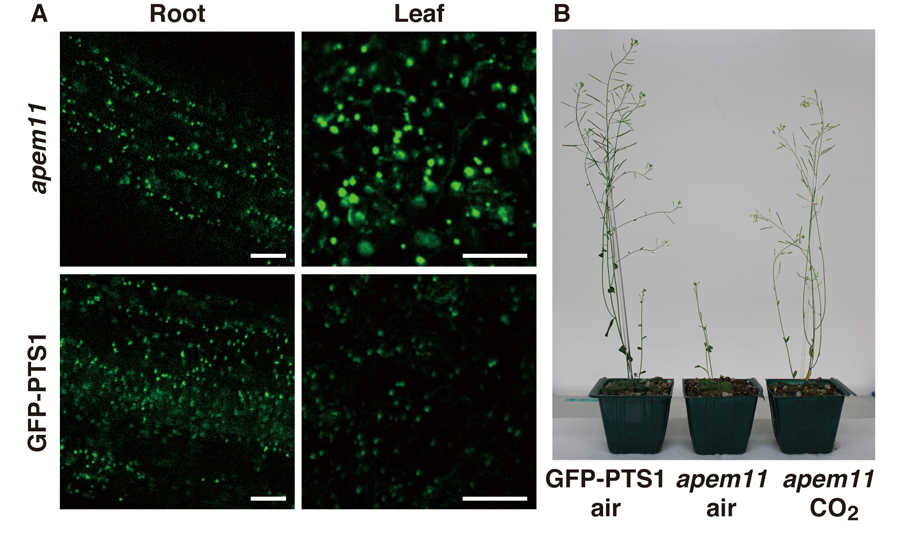
Figure 1. Phenotype of Arabidopsis
apem11 mutants. (A) GFP fluorescence was observed in the parent plant, GFP-PTS1, and
apem11 mutants. The
apem11 mutant has enlarged peroxisomes in leaf cells, but not in root cells. Bars, 20 µm. (B) The
apem11 mutant showed dwarfism under normal atmosphere, but grew normally under high CO2 atmosphere as did the GFP-PTS1 plant.
II. Accumulation mechanism of seed storage oils and proteins
Plant seeds accumulate huge amounts of storage reserves such as oils, carbohydrates and proteins. Humans use these storage reserves in food and industrial materials. Storage reserves vary among different types of plant seeds. Wheat, maize and rice seeds mainly accumulate starch, whereas rapeseed, pumpkin and sesame contain large amounts of oils. Soybeans’ major reserve are proteins. Storage oils and proteins are synthesized in the endoplasmic reticulum (ER) and accumulated in oil and protein bodies, respectively, during seed development.
We are currently analyzing the molecular mechanisms controlling oil and protein contents in seeds (Figure 2). Based on the analysis of the temporal sequence of oil and protein synthesis during seed development in
Arabidopsis thaliana, which produces seeds containing approximately 30% oil and 30% protein, we revealed that the extension of
WRINKLED1 (
WRI1), a transcription factor in fatty acid biosynthesis, expression during the mid-phase of seed development significantly enhanced seed oil content, and caused seed sizes to enlarge.
We are also investigating the mechanisms of oil accumulation in other plant species. In the soybean (
Glycine max. L), we identified four lipases, GmSDP1s, on the oil body membrane. The analyses of GmSDP1s revealed that plant seeds have a mechanism required for the quality control of fatty acids by degrading particular fatty acids in oil bodies.
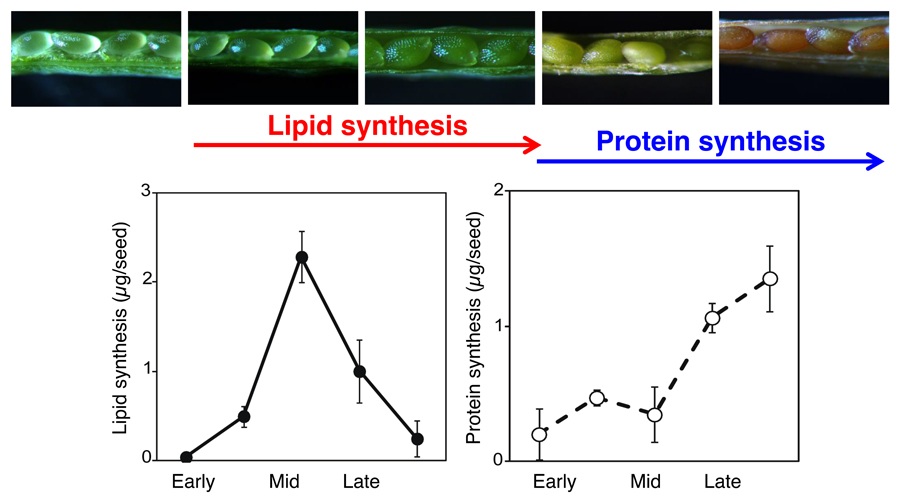
Figure 2. Biosynthesis of lipids and proteins during Arabidopsis seed development. Lipid synthesis and accumulation begins before those of seed storage proteins during seed development.
III. Development of Gateway-technology vectors for plant research
Gateway cloning is a useful and powerful technology which allows the simultaneous generation of multiple constructs containing a range of fusion genes. We have developed various types of Gateway cloning-compatible vectors to improve resources in the plant research field. Up until now, we have provided vector sets to detect multiple protein-protein interactions in vivo using multi-color bimolecular fluorescence complementation, and the binary vectors to facilitate tripartite DNA assembly and promoter analysis with various reporters and tags in the liverwort
Marchantia polymorpha. We will continue developing other useful Gateway cloning-compatible vectors to contribute to the plant research community.
IV. Construction of The Plant Organelles Database 3 (PODB3) and Plant Organelles World
The Plant Organelles Database 3 (PODB3) was built to promote a comprehensive understanding of organelle dynamics (Figure 3), and, as a public database, it is open to all researchers. PODB3 consists of six individual sections: the electron micrograph database, the perceptive organelles database, the organelles movie database, the organellome database, the functional analysis database, and external links. The function of each database is as follows:
・ The electron micrograph database provides information concerning the ultrastructures in plant cells
・ The perceptive organelles database shows organelles dynamics responding to environmental stimuli
・ The organelles movie database contains time-lapse images and 3D structural rotations
・ The organellome database is a compilation of static image data of various tissues of several plant species at different stages of development.
・ The functional analysis database is a collection of protocols for plant organelle research
Through these databases, users can more easily comprehend plant organelle dynamics. Plant Organelles World, which is built based on PODB3, is an educational tool for engaging members of the non-scientific community to explore plant biology. We hope that both PODB3 and Plant Organelles World are of help to researchers as well as the general public.
Figure 3. The graphical user interface of the PODB3 (
http://podb.nibb.ac.jp/Organellome).