Membrane trafficking between single membrane-bound organelles plays an essential role in various activities in eukaryotic cells. Recent comparative genomics has revealed that membrane trafficking pathways have diversified among eukaryotic lineages, with lineage-specific acquisition of new trafficking pathways and secondary loss of pre-existing trafficking pathways. Our long-term goal is to elucidate how plants acquired their unique membrane trafficking systems during evolution. This will be achieved by comparative analyses using the model plant Arabidopsis thaliana and a liverwort model, Marchantia polymorpha. We also aim to elucidate the detailed molecular mechanisms and physiological functions of membrane trafficking in higher-ordered plant functions. In collaboration with other groups with expertise in artificial intelligence, we are also developing a framework to provide new insights into basic biology.
Research Projects
-
• Diversification and evolution of membrane trafficking systems and organelle functions
-
• Molecular mechanisms of endosomal and vacuolar transport in plants
-
• Molecular mechanisms of cargo sorting in membrane trafficking
-
• Comparative analysis of membrane trafficking between Marchantia and Arabidopsis
I. Diversification of membrane trafficking pathways associated with the acquisition of novel machinery components
Although the basic framework of membrane trafficking is well conserved among eukaryotic lineages, recent comparative genomics has suggested that each lineage has acquired unique membrane trafficking pathways during evolution. RAB GTPases and SNARE proteins are evolutionarily conserved key regulators active in the tethering and/or fusion of membrane vesicles with target membranes. It has been proposed that lineage-specific diversification of these key factors is closely associated with the acquisition of lineage-specific membrane trafficking pathways, whose molecular basis remains unknown. Comparisons of these protein families’ organizations among plant lineages, followed by functional analyses of each gene product in A. thaliana and M. polymorpha, indicated that diversification and specialization of membrane trafficking pathways in land plants have been achieved by 1) acquisition of novel machinery components, 2) relocation of conserved machinery components to distinct trafficking events, and 3) secondary loss of conserved machinery components during evolution.
1-1 Analysis of the liverwort-specific organelle: the oil body
The oil body is an organelle specific to liverworts, whose origin and biogenesis mechanism remained unclear for over a hundred years. We are studying the oil body in M. polymorpha, as a model of newly-acquired organelles in specific lineages during evolution. Through comprehensive analyses of SNARE members and organelle markers in M. polymorpha, we identified that a member of the SYP1 group, which generally acts at the plasma membrane in the secretory pathway, was targeted to the oil body. Together with other lines of evidence, we concluded that the oil body is formed by the redirection of the secretory pathway. We additionally proposed the oil body cycle hypothesis; the periodic redirection of the secretory pathway mediates oil body development and growth of the oil body cell. Furthermore, we revealed that the oil body acts as a defense against potential arthropod herbivory by using mutants of the master regulator of oil body formation that we identified, MpERF13.
The morphology and distribution pattern of the oil body, e.g. its shape, color, number, and a density of oil body cells in tissues diverge among liverwort species, which, therefore, are regarded as important features for taxonomical classification in liverworts. However, molecular mechanisms of oil body morphogenesis remain to be determined. We have successfully isolated some mutants with abnormally shaped oil bodies in M. polymorpha, a mutant of which harbors a mutation in a subunit of the COPI coat complex (Figure 1). Given that COPI acts around the Golgi apparatus and plays an essential role in secretion, our finding further supports that the liverwort oil body is formed by the redirection of the secretory pathway.
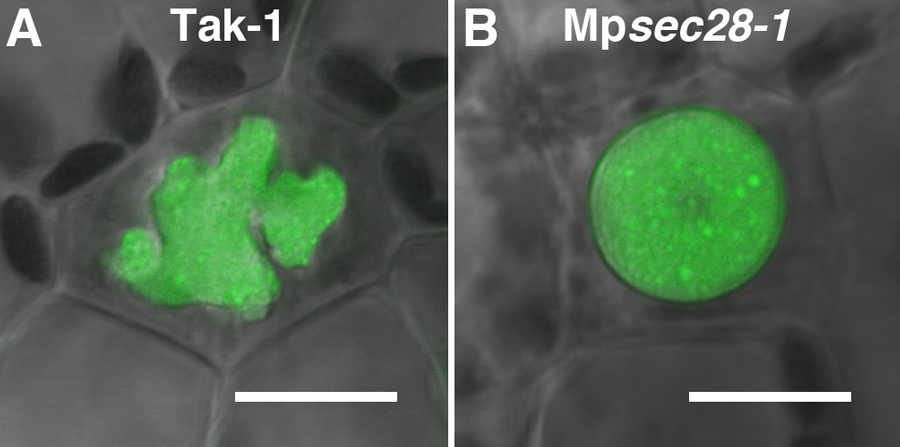
Figure 1. COPI-mediated secretory activity is required for normal oil body formation. The oil body is a complex and bumpy-surfaced structure in wild type (A). In the Mpsec28-1 mutant, the oil body is more rounded with a smoother-surface (B). The oil bodies were visualized using BODIPY 493/503 (green). Bars = 10 μm.
1-2 Mechanisms and dynamics of vacuolar transport
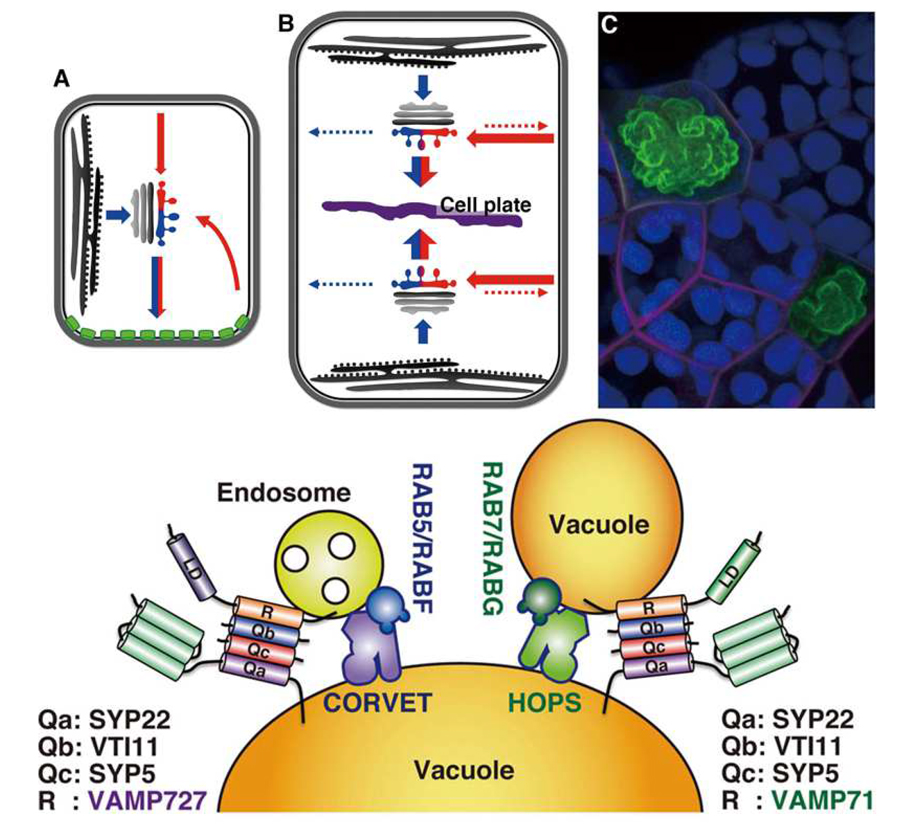
The vacuole is the largest organelle in plant cells, and occupies over 90% of mature plant cells. The vacuole fulfills various functions in plant physiology and development, such as protein degradation, protein storage, and the regulation of turgor pressure. To perform these vacuolar functions, a wide variety of vacuolar proteins and other components must be properly transported to the vacuole, the entirety of which is mediated by membrane trafficking, which is a process distinctly regulated from non-plant systems (for example, Takemoto et al., 2018).
Defective vacuolar SNARE functions affect both vacuolar transport and morphology. The sgr3-1 (shoot gravitropism3) mutant was isolated as a mutant deemed defective in shoot gravitropism. This resulted from a point mutation in SYP22/VAM3, which is one of the SNARE proteins residing on the vacuole and active in vacuolar transport. The sgr3-1 mutant exhibits abnormal vacuolar morphology, although vacuolar transport is not markedly affected in this mutant. We also found that machinery components for homotypic vacuolar membrane fusion including VAMP71, SYP22, and the tethering HOPS complex were accumulated at specific domains in the vacuolar membrane in sgr3-1. These results suggested that vacuolar membrane homotypic fusion is specifically affected by the sgr3-1 mutation.
Plant-specific features of the vacuolar transport were partly achieved by acquisition of a plant-unique SNARE protein, VAMP727. VAMP727 executes membrane fusion between the multivesicular endosome and vacuole, assisted by the CORVET tethering complex. After execution of the membrane fusion, VAMP727 must be recycled back from the vacuolar membrane for sustainable vacuolar transport. However, mechanisms of recycling from the plant vacuolar membrane remain totally unknown. By using VAMP727 as a model cargo, we are currently exploring molecular mechanisms of recycling from the vacuolar membrane. We already succeeded in isolating several candidates that could act in this trafficking pathway.
II. Significance of membrane trafficking in higher-ordered plant functions
2-1 Functions of ANTH-domain proteins in plant physiology
AP180 N-terminal homology domain-containing proteins (ANTH proteins) are thought to act as adaptors bridging the clathrin coat and cargo proteins during clathrin-coated vesicle formation. The ANTH group has been remarkably expanded during land plant evolution, and we are investigating how this protein family has been functionally diversified in A. thaliana. We found that a pair of ANTH proteins, PICALM1a and PICALM1b, are required for retrieving a secretory SNARE protein, VAMP721, from the plasma membrane. This function is required for normal vegetative development of A. thaliana (Figure 2). This finding also highlighted the divergent mechanisms of VAMP7 recycling from the plasma membrane between plants and animals.
We also found that another paralogous set of PICALM proteins, PICALM5a and 5b is required for tip-localization of ANXUR receptor kinases acting in an autocrine signaling pathway required for pollen tube integrity in A. thaliana (Figure 2). Thus, functionally differentiated ANTH proteins underpin various physiological processes in A. thaliana.
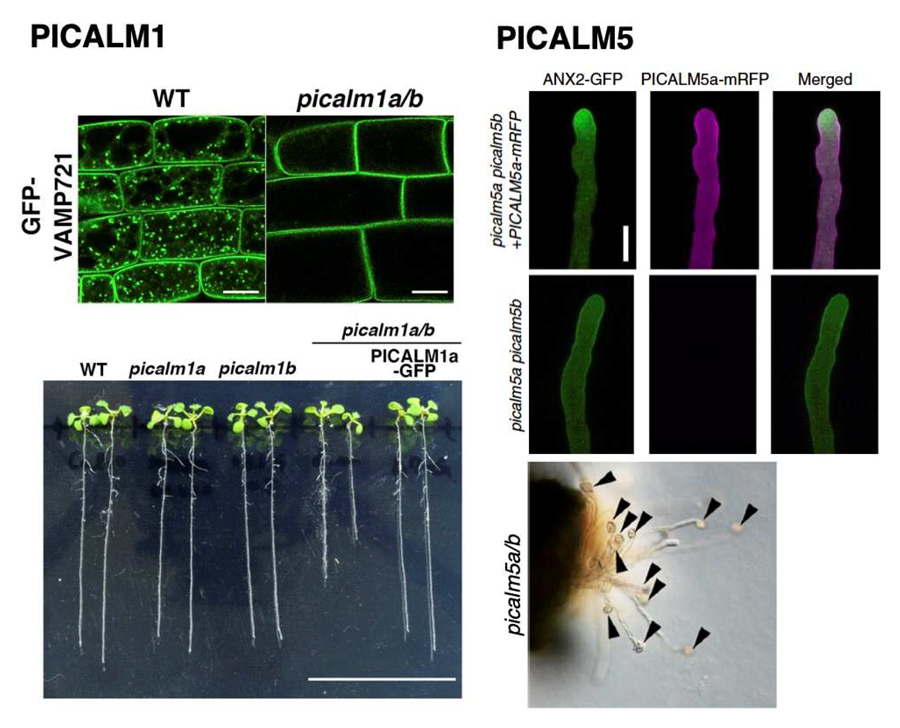
Figure 2. PICALM1 and PICALM5 mediate endocytosis of distinct plasma membrane proteins, which are required for normal vegetative development and pollen tube integrity, respectively. (Left: Fujimoto et al., 2020, modified, right: Muro et al., 2018, modified)
In addition to PICALM5, PICALM2, PICALM6, and PICALM9 are also highly expressed in A. thaliana pollen and/or pollen tubes, which suggests that these PICALMs underpin the complex endocytosis system in pollen tubes. Recently, we found that the picalm2a/2b double mutant exhibits defective transmission of the mutation from male plants, which suggested that PICALM2 acts in endocytic transport of important but still unknown cargo(s) in pollen and/or pollen tubes. Now we are investigating molecular mechanisms of endocytosis in pollen tubes with a special focus on clathrin adaptor proteins including PICALM members.
Our results indicate that each PICALM protein regulates endocytosis of distinctive cargo proteins, whose molecular mechanisms remain mostly unclear in plants. We are now investigating the molecular function of PICALMs with a special focus on the mechanisms of cargo recognition by PICALMs in A. thaliana.
2-2 Membrane trafficking in plant gametogenesis
Gametogenesis in plants also involves membrane trafficking-mediated processes. We are analyzing molecular mechanisms of gametogenesis in A. thaliana and M. polymorpha, and are focusing our attention on secretory and degradative trafficking pathways during male gamete formation in particular.
Cytokinesis in land plants is achieved by the re-direction of the secretory pathway. As such, KNOLLE/SYP111 plays an important role in membrane fusion in the formation of cell plates in A. thaliana somatic cells. Conversely, no deleterious effects on gametogenesis have been reported regarding mutations in KNOLLE. We found that KNOLLE and other SYP1 members were highly expressed during cytokinesis in gametogenesis (Figure 3). Mutant analyses of syp1 members also supported that KNOLLE and other SYP1 regulate cytokinesis during gametogenesis in A. thaliana.
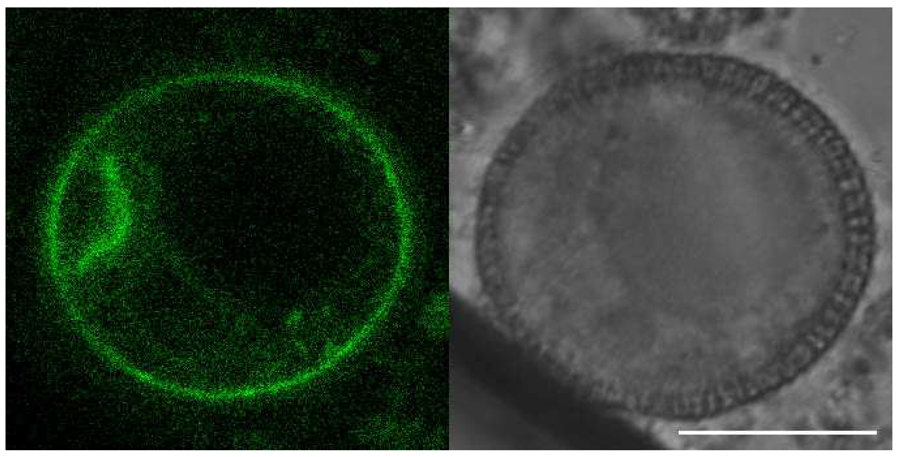
Figure 3. Expression and subcellular localization of GFP-KNOLLE during pollen mitosis I. GFP-KNOLLE accumulates at the cell plate. Bar = 10 μm.
Distinct from seed plants, basal land plants including M. polymorpha utilize the spermatozoid with two (or more) motile flagella as the male gamete during sexual reproduction. We visualized the spermatozoid formation process, especially spermiogenesis, using fluorescently-tagged organelle markers in M. polymorpha. The majority of the endomembranous organelles, such as the Golgi apparatus, were removed from maturing spermatozoid cells, and the plasma membrane was also reorganized during spermiogenesis. Inspection by transmission electron microscope and live-cell imaging analyses also indicated that the number of degradative organelles such as the multivesicular endosome, vacuole, and autophagosome, is transiently increased during this process. To reveal the molecular mechanisms of cytoplasm removal and organelle remodeling, we have established the analytical tools of autophagy in M. polymorpha (Norizuki et al., 2019). M. polymorpha possesses core machineries of autophagy with lower degrees of redundancy. The mutations in MpATG5 and MpATG7, which are key factors for autophagosome formation, affected the transportation of cytosolic components to the vacuole for degradation.
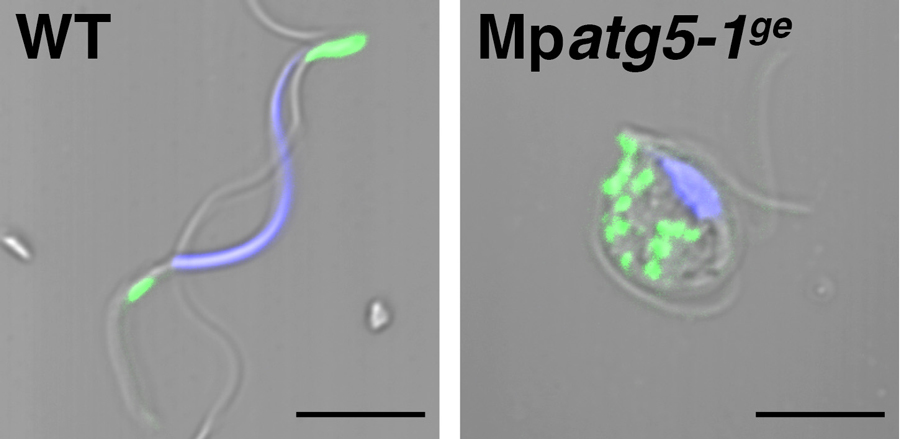
Figure 4. Impaired reorganization of mitochondria in an autophagy-defective mutant of M. polymorpha. The wild-type spermatozoid possesses two mitochondria (green), whereas a number of mitochondria remained in the Mpatg5-1ge mutant spermatozoid. Blue: nuclei. Bars = 5 μm.
Autophagy-defective mutants exhibited defects regarding cytoplasm removal, spermatozoid motility, and fertility. Although a majority of organelles are removed during spermiogenesis, a specific set of organelles, e.g. anterior and posterior mitochondria, persists in mature spermatozoids (Figure 4), which implies that there should be a mechanism for selective removal of unneeded organelles. By analyzing the effect of defective autophagy during spermiogenesis in M. polymorpha, we found that spermiogenesis occurs through multimodal autophagic degradation.
We also found that spermiogenesis requires the proper function of the ESCRTs complex, which mediates multivesicular endosome formation and sorting of membrane proteins to be degraded. These results indicate that highly organized autophagic and non-autophagic degradation play crucial roles to accomplish spermiogenesis in M. polymorpha.
We are also analyzing the role of RAB GTPases in flagella formation. Through a comprehensive analysis of RAB GTPases in M. polymorpha, we found that a RAB GTPase plays an essential role in generating fully functional flagella (Figure 5).
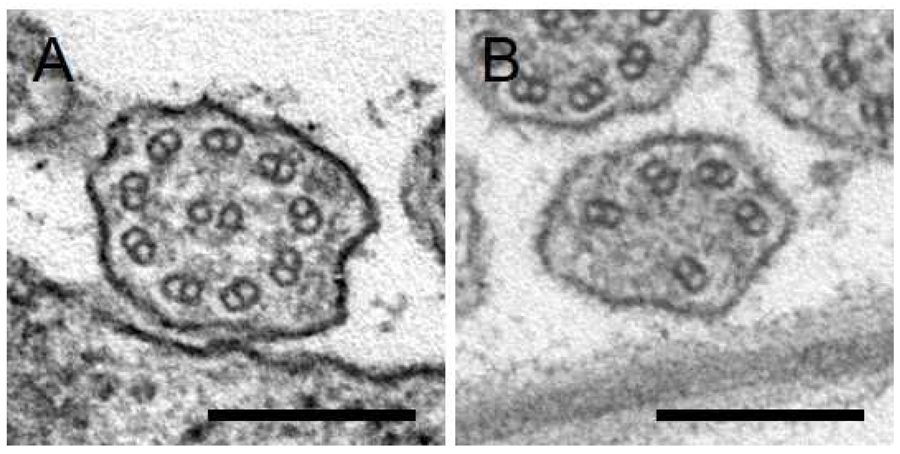
Figure 5. Transverse sections of flagella in wild-type (A) and mutant (B) spermatids. The microtubule-based “9 + 2” axoneme structure is severely compromised in the mutant. Bars = 200 nm.











