Plant organs have the ability to sense various vectorial stimuli such as light, humidity, touch, and gravity as well as reorient their growth direction so as to be in a suitable position to survive and acclimate to their environment. Our aim is to understand the molecular mechanisms of environmental responses related to gravity.
Research Projects
-
• Molecular mechanisms of gravity signaling in gravity-sensing cells
-
− Mechanism of amyloplast localization of LZY, a plant-specific membrane-associated protein that functions as a gravity-signaling molecule
-
− Mechanism of LZY translocation from amyloplasts to the plasma membrane
-
− Gravity-responsive auxin transport regulatory platform formed by LZY-RLD
-
• Gravitropic setpoint angle (GSA) control mechanism of lateral branches
-
• Statolith-independent gravity sensing mechanism
I. Molecular mechanisms of gravity sensing and signaling in gravitropism
Gravitropism is a major determinant in directing plant organ growth angles. In gravity sensing cells (statocytes), plastids accumulating starch in high-densities relocate toward the direction of gravity. Amyloplast relocation serves as a physical signal trigger for biochemical signal transduction, which in turn leads to the regulation of the polar auxin transport necessary to change the direction that a given plant is growing. We are investigating the detailed molecular mechanism of gravity sensing and signaling by using the model plant
Arabidopsis thaliana.
LAZY1 family genes have been shown to be involved in gravitropic responses in a variety of plants.
LAZY1-LIKE (
LZY)2, LZY3, and
LZY4 are involved in root gravitropism of Arabidopsis. Previously, we have found that LZY3-mCherry is polarly localized on the basal side of the plasma membrane in the columella cells (root statocytes) in response to inclination. This suggests the close relationship between intracellular localization of LZY and gravity sensing. To elucidate the gravity sensing mechanism, we are analyzing regions responsible for intracellular localization of LZY3-mCherry and LZY4-mSacarlet by introducing mutations. A number of transgenic lines harbouring mutated LZY-FP driven by their own promoters in mutant background are under construction.
II. Determination mechanism of gravitropic setpoint angle
Growth angles affected by gravity are known as the gravitropic setpoint angles (GSA). Many gravitropic mutants show abnormal GSA in lateral branches; meaning they produce wider growth angle phenotypes due to the likelihood of reduced gravitropism. We are trying to understand how roots’ and shoots’ lateral branches maintain inclined growth angles with respect to gravity.
It has been proposed that the GSA is determined by two antagonistic growth components, gravitropism and anti-gravitropic offset (AGO). Currently, we are characterizing Arabidopsis
TAC1 (
TILLER ANGLE CONTROL 1) gene as a candidate to control AGO. The
TAC1 gene was originally identified by quantitative trait locus analysis in rice tiller angle control (Yu
et al., 2007). Later,
TAC1 gene was identified in Peach tree (
Prunus persica) as a causal gene of piller phenotype (Dardick
et al., 2013). In our lab,
TAC1 gene was found as a down regulated gene in agravitropic mutants
eal1 and
sgr1 which lack an endodermis, a gravity sensing tissue in shoot in Arabidopsis (Taniguchi
et al., 2017). In Arabidopsis,
tac1 mutant has a compact shoot architecture due to the upward lateral branch growth angles (Figure 1).
TAC1 encodes a protein with unknown function, but has a similarity with LAZY1-LIKE family protein. However, while LZY family proteins interact with RLD family proteins through their conserved C-terminus in LAZY1 family protein (CCL) domain and regulate gravitropism (Furutani
et al., 2020), TAC1 does not have a CCL domain, thereby TAC1 does not interact with RLD family protein.
As well as characterizing
TAC1 gene, currently, we are also trying to isolate suppressor mutants of
lzy1 lzy2 lzy3 by a forward genetic screening.
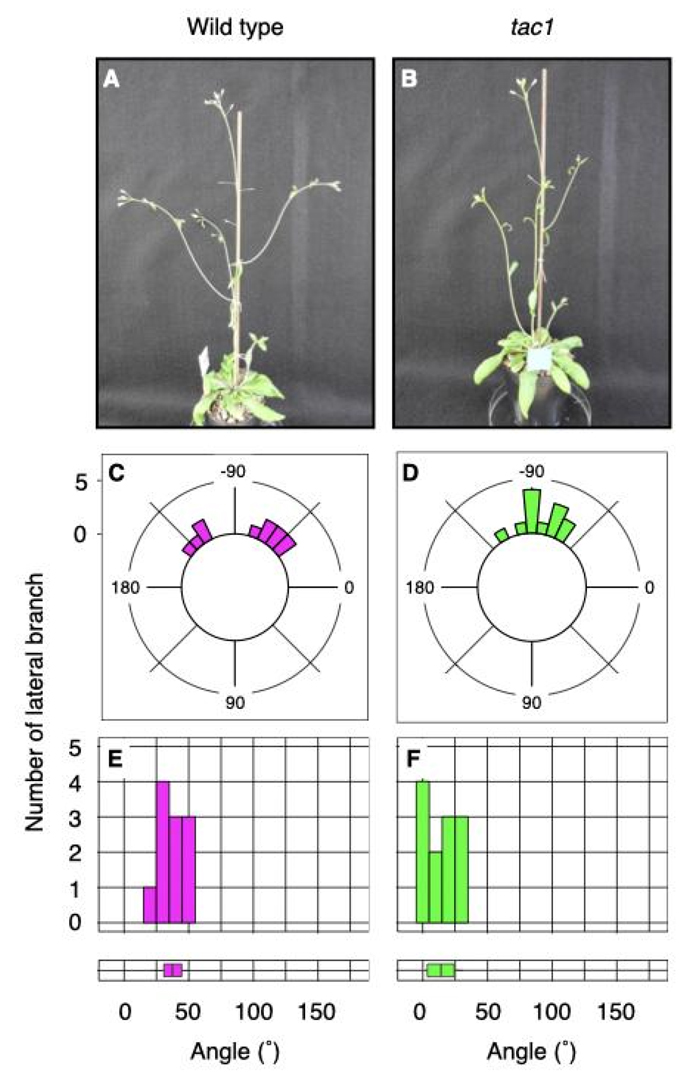
Figure 1. Growth angle phenotype of shoot lateral branches. Overall shoot architecture in wild type (A) and
tac1 (B). Distribution of growth direction of lateral branches in wild type (C) and
tac1 (D). Growth angles were plotted on histogram and distributions were evaluated in boxplot from wild type (E) and
tac1 (F).
III. Functional analysis of RLD (RCC1-like domain) proteins
RLD proteins were isolated as interactors of LZY. RLDs function in gravitropism and the GSA control of roots. We have also shown that
RLD1, RLD2, RLD3, and
RLD4 redundantly function in plant development and morphogenesis, the morphological abnormality gradually becomes more severe by multiplying
rld mutations (Figure 3). Indeed,
rld1;2;3;4 quadruple mutants displayed severe embryonic development defects due to a reduced amount and abnormal localization of PIN proteins (auxin efflux carriers) in the plasma membrane. It had been reported that the RLD1 fragment containing RCC1-like domain show guanine nucleotide exchange activity in Rab8 and Rab11. Thus, it’s likely that RLD proteins regulate auxin transport through regulating membrane trafficking during root gravitropism and development.
In columella cells, RLD1 is polarly recruited to the plasma membrane by interacting with LZY3 and regulates the direction of auxin transport. The interaction between RLD1 and LZY3 occurs through the binding of the BRX domain of RLD1 to the CCL domain of LZY3.
To understand further function of RLDs, we made attempt to identify the proteins that interact with RLD using yeast two-hybrid screening. As a result, we found several proteins as candidates for RLD interactor in addition to LZYs. Among them, we further analyzed the polarity protein BASL (BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE) which controls stomatal asymmetric cell division as a collaboration with Dr. Dong and colleagues (Wang
et al., 2021). Further analyses using yeast two-hybrid system revealed that BASL interact with the BRX domain of RLD. Developmental defects of
rld1;2;3;4 mutant phenocopy those of the severe allele of gnome mutants as previously reported (Furutani
et al., 2020). GNOM, a guanine nucleotide exchange factor for ARF (ADP ribosylation factor), plays important roles in membrane trafficking. Dong and colleagues found that RLD physically interacts with GNOM in vitro and in vivo, and that GNOM and RLDs are required for BASL polarization. Interestingly, we found GNOM in the immunoprecipitates with LZY proteins (Furutani
et al., 2020), implying that RLD-GNOM could be involved in LZY function in the gravity signaling.
To investigate the importance of the BRX domain for RLD function, we examined whether
RLD3p:RLD3DBRX-mClover3 could complement the morphological phenotype of
rld2;3;4. Unexpectedly, RLD3DBRX-mClover3 recovered the morphological abnormality of
rld2;3;4 (Figure 2). Interestingly, defects in gravity response including GSA control and gravitropism were observed in
RLD3p:RLD3DBRX-mClover3/rld2;3;4. Both of these indicate that RLD3 lacking the BRX domain retains the function for morphogenesis but not for gravity response in shoot. Moreover, when RLD3 was expressed under the control of the endodermis-specific ADF9 promoter in
RLD3p:RLD3DBRX-mClover3/rld2;3;4, the gravity response was significantly restored, thus suggesting that RLD is involved in gravity signaling in the endodermal cells in shoot gravitropism in a manner similar to LZY. These results suggest that RLD function in several physiological events including gravitropism, morphogenesis in BRX-dependent and -independent manner.

Figure 2.
A. Phenotype of
rld multiple mutants in mature plants.
B. The growth angle of lateral shoot in Wild Type (a),
RLD3p:RLD3DBRX-mClover3/
rld2;3;4 (b),
ADF9p:RLD3/
RLD3p:RLD3DBRX-mClover3/
rld2;3;4 (c).
IV. Optogenetic approaches in plant biology
Optogenetics is a powerful, biological technique to manipulate the activity and localization of proteins in living cells, thereby understanding their function. Many optogenetic tools have been developed for the past three decades, however there are few applications in plant biology because of biological and technical limitations; 1) plants use light for photosynthesis or as their development cues and 2) most of the tools are developed from plant light-sensitive proteins, in which light stimulating the tools may affect plant growth and development in an undesirable manner. Even so, optogenetic approaches are worth introducing into plant biology because they can control proteins of interest with a higher spatiotemporal resolution than those of conventional techniques like gene-inducible system and protein-uncaging with chemicals. We have been trying to optically control plant protein kinases, particularly belonging to AGC kinase family, which regulate fundamental cellular processes in plant growth and development, such as transport of plant hormone auxin and cell growth. We chose a strategy to control the kinase activity directly by light, which can be controllable at the subcellular level. To carry out it, we fused light-responsive proteins/modules to the kinases and analyzed their responsibility to light. One of successful tools possess a blue light-sensitive LOV2 domain from plant photoreceptor phototropin and its interacting motif that we identified to negatively regulate AGC kinase activity by blue light. By using this tool, we succeeded to develop a light-sensitive kinase that directly regulates the auxin transporter PINs, which is proven at least by a heterogenous expression system with
Xenopus oocytes. It is expected that this tool will be a breakthrough technique in plant biology to dissect lots of biological events mediated by auxin (Figure 3).
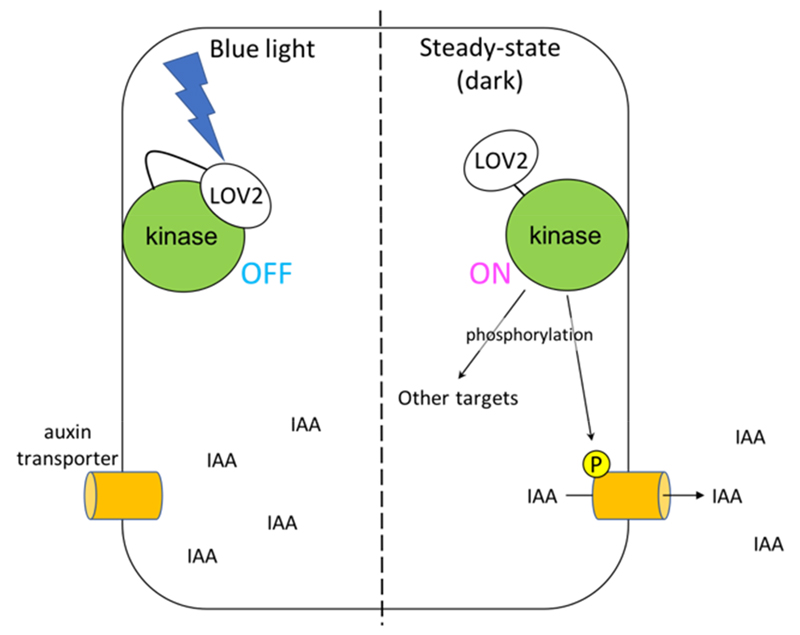
Figure 3. Schematic illustration of the light-sensitive AGC kinase. In the dark, the chimeric AGC kinase is active to phosphorylate its target proteins such as auxin transporter PINs (right). PINs are activated by the phosphorylation for efflux transport of indole-3-acetic acid (IAA). Upon blue light irradiation, the kinase activity can be reduced due to the blue light-induced conformational change (left).