We would like to know key genetic changes that resulted in the evolution of influential novel traits in land plants. For this purpose, we select new key-stone model organisms, sequence the genomes, establish genome editing techniques, and investigate mechanistic novelty at both cellular and organismal levels.
Research Projects
-
• molecular mechanisms and evolution of rapid signal transduction in plants with electrical and calcium signals
-
• reprogramming from somatic cells to pluripotent stem cells
-
• evolution of development and morphology in terrestrial plants
I. Evolution of Complex Adaptive Characters
The theory of natural selection and the neutral theory of molecular evolution are fundamental concepts in evolutionary biology. However, even with such theories, there still remain unexplained phenomena, one of which is the evolution of complexity. It is difficult to explain the mechanisms needed to evolve complex adaptive traits, because such traits comprise plural components and become adaptive only when all components are gathered together. However, based on evolutionary theories, each component should evolve one by one according to the accumulation of mutations.
To understand the molecular and cellular bases of adaptive traits, we study the molecular and cellular mechanisms and evolution of (1) long distance signaling in plants, (2) reprogramming from differentiated cells to stem cells, and (3) development and morphology in land plants.
II. Molecular mechanisms and evolution of long distance signaling in plants
Plants lack blood flow and nerves, but have evolved unique long-distance intercellular signaling mechanisms. Signals using plant hormones, peptides, proteins, and slow calcium waves have been well elucidated; however, the molecular mechanism of long-range, rapid, intercellular signaling by action potentials with fast calcium waves, which evolved in parallel to similar signaling mechanisms in animals, remains largely unknown. Rapid transmission of action potentials has been reported in specific tissues of the sensitive plant Mimosa pudica, the Venus flytrap Dionaea muscipula, and the sundew Drosera rotundifolia. We use these species, as well as Arabidopsis thaliana, to study the molecular mechanisms and evolution of action potential generation and transmission. We have obtained genome sequences for these species (Palfalvi et al. 2020 Curr. Biol. for Dionaea and unpublished for Mimosa pudica and Drosera rotundifolia) and established techniques for transformation (Mano et al. 2014 PLoS ONE for Mimosa pudica; Suda et al. 2020 Nat. Plants for Dionaea; unpublished for Drosera rotundifolia). We intend to screen for genetic factors responsible for fast intracellular and intercellular electrical signaling by action potentials, analyze their molecular characteristics such as effects on ion permeability and intracellular localization, and perform genetic gain- and loss-of-function experiments. This will allow us to understand the general mechanisms of action potential transmission in plants and the evolutionary process that resulted in diversity of transmission velocity, which is adaptive in the three plants.
II-1. Molecular mechanisms of long distance signaling of mechano-stimuli in the sensitive plant Mimosa pudica
The sensitive plant Mimosa pudica folds it leaves within a couple of seconds in response to touch. These rapid movements are mediated by specialized motor organs, pulvini, which locate at the base of each compound leaves, pinnae, and small leaflets. When getting stimulated, motor cells in one side of the pulvinus (extensor half) shrink while cells in the opposite side (flexor half) do not shrink, consequently leading to the unidirectional bending of the pulvinus. Because a pulvinus contains thousands of motor cells, achievement of the rapid movement at a whole pulvini requires not only the rapid shrinkage of individual motor cells but also rapid synchronization mechanisms among them. The molecular mechanisms underlying these rapid shrinkage and rapid cell-cell communication still remain open questions.
To elucidate the molecular mechanisms for rapid leaf movements, we have developed an efficient transformation technique in M. pudica, and then established in planta live imaging of Ca2+ with sensor fluorescent proteins and CRISPR/Cas9-mediated gene knockout. To obtain candidate genes responsible for rapid movements, we compared gene expression profiles between pulvini and non-motor organs, and between extensor and flexor halves of pulvini. As a result, we got a number of extensor-enriched genes. After the systematic knockout experiments of these candidate genes, mutants of two channel genes, one gene encoding a membrane-associated protein with uncharacterized functions, and one transcription factor showed deficiencies in touch-induced rapid movements. Currently we are working on the detailed analysis on functions of those genes and mutans by using transgenic techniques and electrophysiological studies employing a heterologous expression system in Xenopus oocytes.
Rapid movements in M .pudica take only 1 to 2 seconds for the whole process and our preliminary observations suggested that their initial events like Ca2+ increase occurred within 100 ms or less. To achieve higher time resolution for our fluorescent live imaging, we introduced a high-speed and high-sensitivity camera system, and developed a method to analyze spatio-temporal propagation of Ca2+ signals robustly. We found that in the tertiary pulvinus, a mechanical stimulus applied at the center evokes a rapid concentric propagation of Ca2+ wave with 1.5 cm/s velocity, and it only took 30 ms to reach to the outermost edge of excitable motor cells in the extensor half (Figure 1). These analytical methods will facilitate future studies using mutant lines described above. This study was mainly performed by Dr. Hiroaki Mano with the support by technical staff.
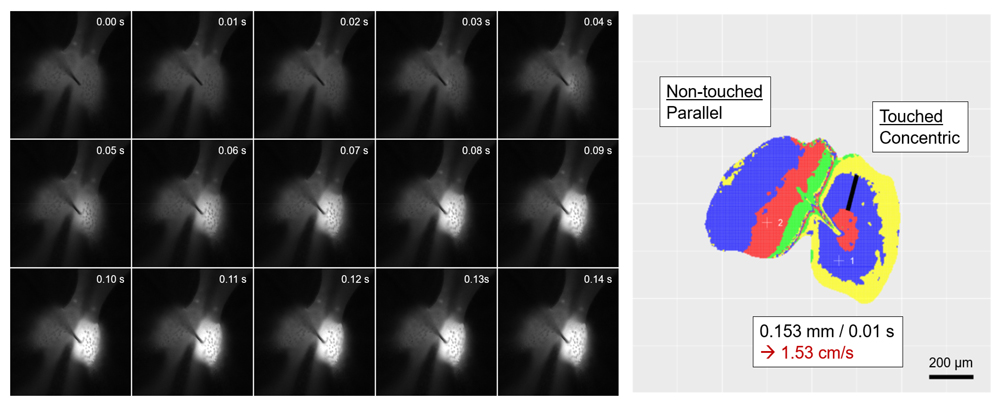
Figure 1. Fluorescence images of a red Ca2+ sensor R-GECO in a M. pudica tertiary pulvinus after stimulated with a needle (100 fps). Right image shows how the wave front propagates. 4 colors are repeatedly used (R-B-Y-G) to discriminate each 10 ms frame.
II-2. Molecular mechanisms and evolution of the generation and transmission of action potential in the sundew Drosera rotundifolia and the Venus flytrap Dionaea muscipula
Drosera rotundifolia is a carnivorous plant that captures prey for nutrients by its tentacles, multicellular hairs on the leaf with sticky digestive fluid at their heads. Each tentacle senses mechano- and chemical-stimuli at its head, and cells in a tentacle base bend to bring the captured prey to the center of the trap leaf. In this process, action potential is elicited at the head by the stimulus and transmits through the stalk cells to the basal cells. The advantage of D. rotundifolia to study transmission mechanisms of action potential is in the simple structure of the tentacle, which is feasible for electro-physiology and bio-imaging. We succeeded to cultivate D. rotundifolia under aseptic conditions with proper reactions to the stimuli, and furthermore, to transform D. rotundifolia with the green fluorescent protein gene. We improved the transformation efficiencies with optimizing nutrient conditions in the medium.
In this fiscal year, nuclear genome of D. rotundifolia was sequenced with long-read sequencing with Nanopore sequencer and linked-read sequencing with TELL-seq library and Illumina sequencer in 50 times coverage. To look for candidate genes to function in the generation and transmission of action potential, we compared mRNA profiles between different tissues in tentacles and between tentacles and laminas. Loss-of-function experiments with CRISPR/Cas9 mediated gene knockout are ongoing.
Ca2+ sensor RGECO expressed transformants were established to investigate the relationship between rapid calcium wave and action potential transmission. Mechanical stimulation to the tentacle head produced a fast Ca2+ wave with similar speed to the action potential, which was transmitted to the basal cells, but not to laminar cells. We further investigate the molecular and cellular mechanisms with other transgenic lines.
The Venus flytrap is another carnivorous plant, whose leaf blade bends to capture prey with two successive mechanical stimuli applied to the sensory hairs on the surface. The mechanical stimulus is sensed at the middle cells of the hair, in which action potential is generated to transmit to whole laminar cells. As done in D. rotundifolia, we looked for candidate genes with comparative transcriptomics, and gene knockout experiments are ongoing. This study was mainly conducted by Dr. Shoji Segami, Ms. Shoko Ooi, Mr. Peng Chen, Mr. Kuniaki Tanase, and Mr. Riku Matsuda with a the support of technical staff. This work is collaboration work with Drs. Hiraku Suda and Masatsugu Toyota in Saitama University.
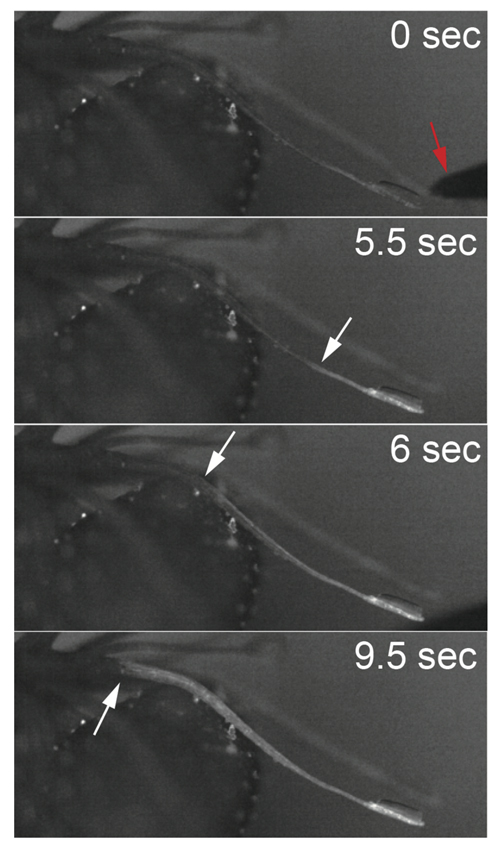
Figure 2. Fluorescence images of a RGECO Drosera rotundifolia tentacle after the tip was stimulated with a needle (red arrow in 0 sec), showing changes of cytosolic calcium ion amounts. White arrows indicate fronts of calcium waves.
III. Molecular mechanisms of reprogramming from differentiated cells to stem cells by STEMIN
Stem cells self-renew and give rise to differentiated cells to form tissues or organs. Differentiated cells in land plants can be readily reprogrammed into stem cells during post-embryonic development and regeneration. Thus, land plants have an intrinsic ability to initiate a new genetic regulatory network, despite the existence of epigenetic memory in differentiated cells. The underlying molecular mechanism and the changes in the network due to mutation in its components contribute to the environmental adaptability of land plants to survive under harsh environments and to the evolution of complex adaptive traits.
To understand the molecular basis of reprogramming, we have used the moss Physcomitrium patens, in which leaf cells can be reprogrammed into chloronema apical stem cells by wounding. During this process, the expression of STEMIN1 gene, which encodes AP2/ERF transcription factor, is activated at leaf cells facing the cut. Deletion of STEMIN1 and its two homologs retards reprogramming, while ectopic STEMIN1 induction changes differentiated cells into chloronema apical stem cells. We also found that transient DNA damage induces reprogramming of leaf cells into stem cells, which requires the expression of STEMIN genes. Thus, STEMIN genes play a role in both the wounding- and the DNA damage-induced cellular reprogramming (Figure 3).
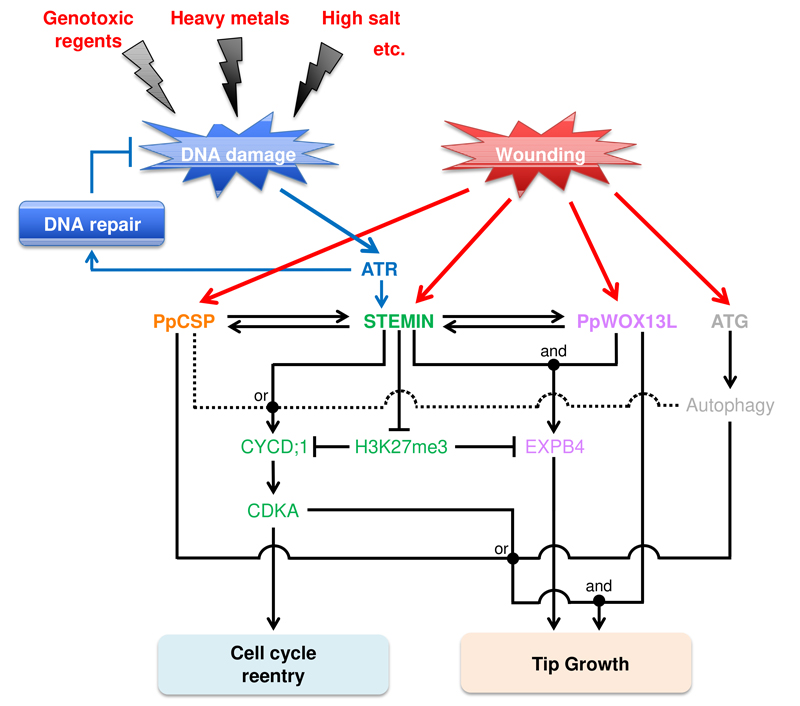
Figure 3. A working hypothesis of a genetic regulatory network of cellular reprogramming in Physcomitrium patens.
We found that ectopic induction of STEMIN1 in leaf cells decreases a repressive chromatin mark, histone H3 lysine 27 trimethylation (H3K27me3), on its direct target genes. To elucidate the mechanism regulating the local H3K27me3 reprogramming, we screened for STEMIN1-interacting proteins and found that some of the components have the potential to function in the histone modification. These suggest that STEMIN1 could have a role as a platform to recruit components needed for local H3K27me3 reprogramming at the specific loci of the genome. This study was mainly performed by Dr. Masaki Ishikawa and Mr. Ruan De Villiers with the support of the technical staff.
IV. Evolution of Development and morphology in land plants
Expansion of individual cells contributes to the morphogenesis of multicellular organisms, especially in land plants, in which cell migration is restricted by the rigid cell wall. Cell walls are mainly composed of the polysaccharides and the outer lipid cuticle layer. Previous reports showed that mechanisms acting on the cell wall polysaccharides, such as cellulose and pectin, regulate cell expansion. However, how other factors regulate cell expansion is not clear. We found that an ATP-binding cassette (ABC) B family transporter, PpABCB14, localized on the plasma membrane of the expanding region of gametophore initial cells and the leaf cells. Mr. Liechi Zhang and Dr. Masaki Ishikawa mainly investigate the molecular mechanisms with support of the technical staff.









