Seed plants, such as soybeans, store large amounts of protein in specialized organelles called protein storage vacuoles. These proteins not only sustain seedling growth but also serve as a major source of dietary protein for humans and livestock. This protein storage function is unique to plant vacuoles and does not occur in other organisms. During evolution, plants developed a dedicated mechanism to transport large quantities of proteins into vacuoles — but how did this transport pathway, which is absent in animals or fungi, come into existence?
In a recent study published in
Current Biology, a research team led by Professor Takashi Ueda of the National Institute for Basic Biology and Associate Professor Masaru Fujimoto of the University of Tokyo has revealed the molecular steps that led to the emergence of this plant-specific vacuolar transport system. Their work shows that the acquisition of this pathway was driven by the stepwise neofunctionalization of a membrane fusion protein called VAMP7.
The team discovered that the plant vacuolar transport system arose through a series of molecular innovations: the appearance of an amino acid insertion within the VAMP7 protein, its acidification over time, and the progressive acquisition and strengthening of binding capacity to the AP-4 adaptor protein complex, which is responsible for cargo selection (Figure 1). These modifications enabled the establishment of a vacuolar transport route unique to seed plants.
Plants contain two major types of VAMP7 proteins: VAMP71, which functions in vacuolar membrane fusion, and VAMP72, which is involved in trafficking to the plasma membrane — part of the secretory pathway.
Professor Ueda, who led the study, explains:
“In addition to these, we previously identified a unique VAMP72-like protein, called VAMP727, in seed plants such as
Arabidopsis thaliana. Unlike typical VAMP72s, VAMP727 contains an acidic amino acid-rich insertion of about 20 residues in the N-terminal longin domain. This protein is known to function in vacuolar trafficking and is essential for transporting storage proteins into vacuoles during seed development. However, it was unclear when and how VAMP727 and its associated trafficking pathway emerged during evolution, or what molecular mechanisms underpinned this innovation.”
To address this, the team focused on the molecular evolution of VAMP7. By comparing the amino acid sequences and functions of VAMP72 proteins across a wide range of plant species, from green algae to seed plants (Figure 2), they reconstructed the evolutionary trajectory by which VAMP72, originally functioning in secretion, acquired a vacuolar trafficking role. Based on these insights, they investigated how specific changes in the VAMP72 sequence altered its localization, function, and protein-protein interactions within cells.
Associate Professor Fujimoto, first author of the study, explains:
“Our findings suggest that VAMP72 first gained a partially acidic insertion containing a tyrosine-based motif, enabling a partial functional shift. As this insertion became more acidic and formed an acidic dileucine motif, the protein's interaction with the AP-4 complex was strengthened, resulting in the emergence of VAMP727, a vacuole-targeted VAMP (Figure 3). We believe that this two-step functional transition led to the birth of a new vacuolar transport pathway in plants, allowing for the massive accumulation of storage proteins in seed protein storage vacuoles.”
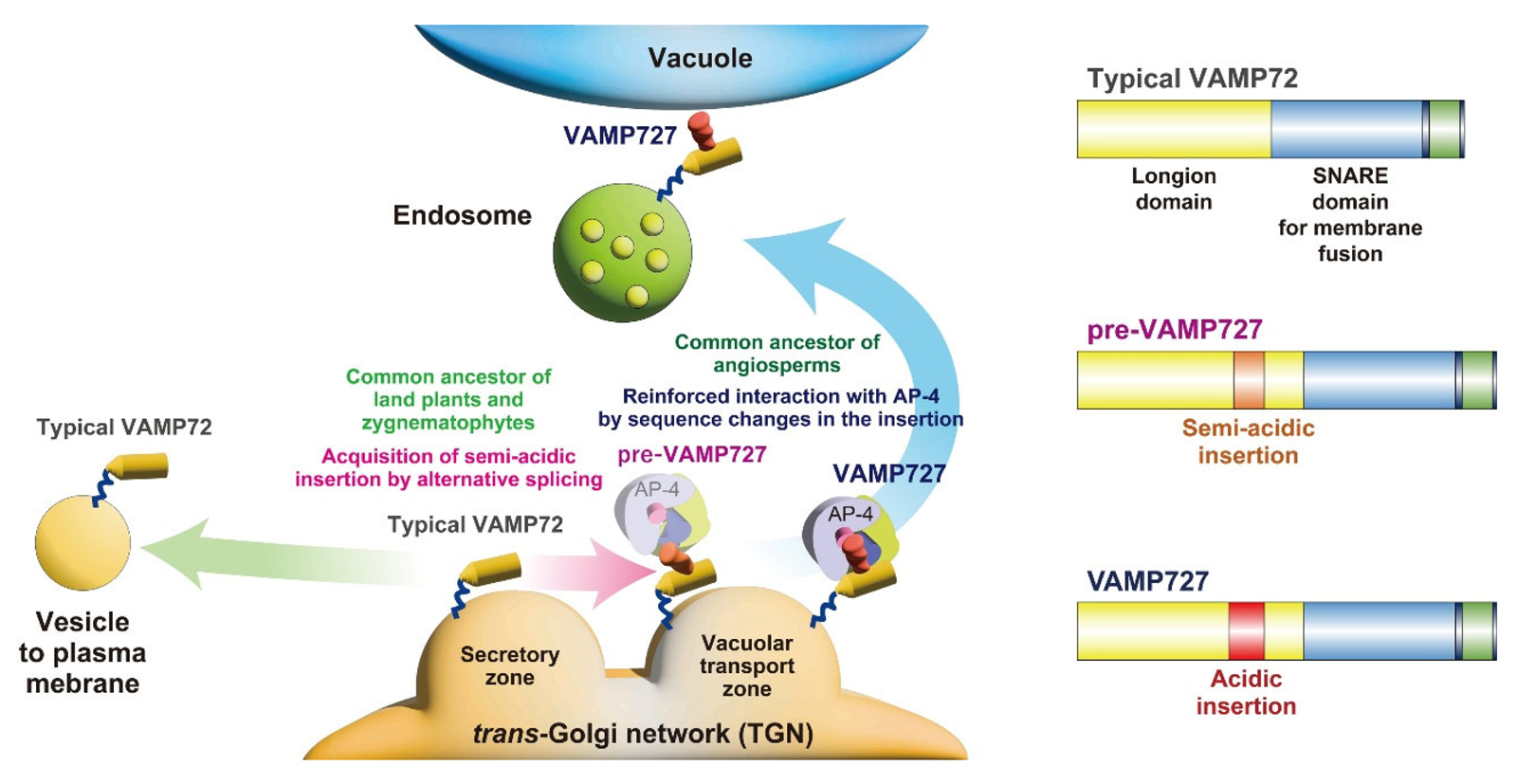 Figure 1. How VAMP72 evolved into VAMP727 through stepwise changes in plants
Figure 1. How VAMP72 evolved into VAMP727 through stepwise changes in plants
This illustration shows how the membrane trafficking protein VAMP72 gradually changed during plant evolution to give rise to VAMP727, which functions in transporting storage proteins to vacuoles. The first step was the acquisition of a short insertion in its structure through alternative splicing. This insertion contained a non-canonical tyrosine-based motif, which enabled a partial shift in localization from the secretory to the vacuolar transport zone within the trans-Golgi network. Later, the insertion became more acidic and acquired a dileucine-like motif, enhancing its interaction with the AP-4 adaptor complex. These stepwise changes allowed VAMP72 to evolve into VAMP727, supporting the development of the efficient vacuolar transport system found in seed plants.
Created by Takashi Ueda and Masaru Fujimoto.
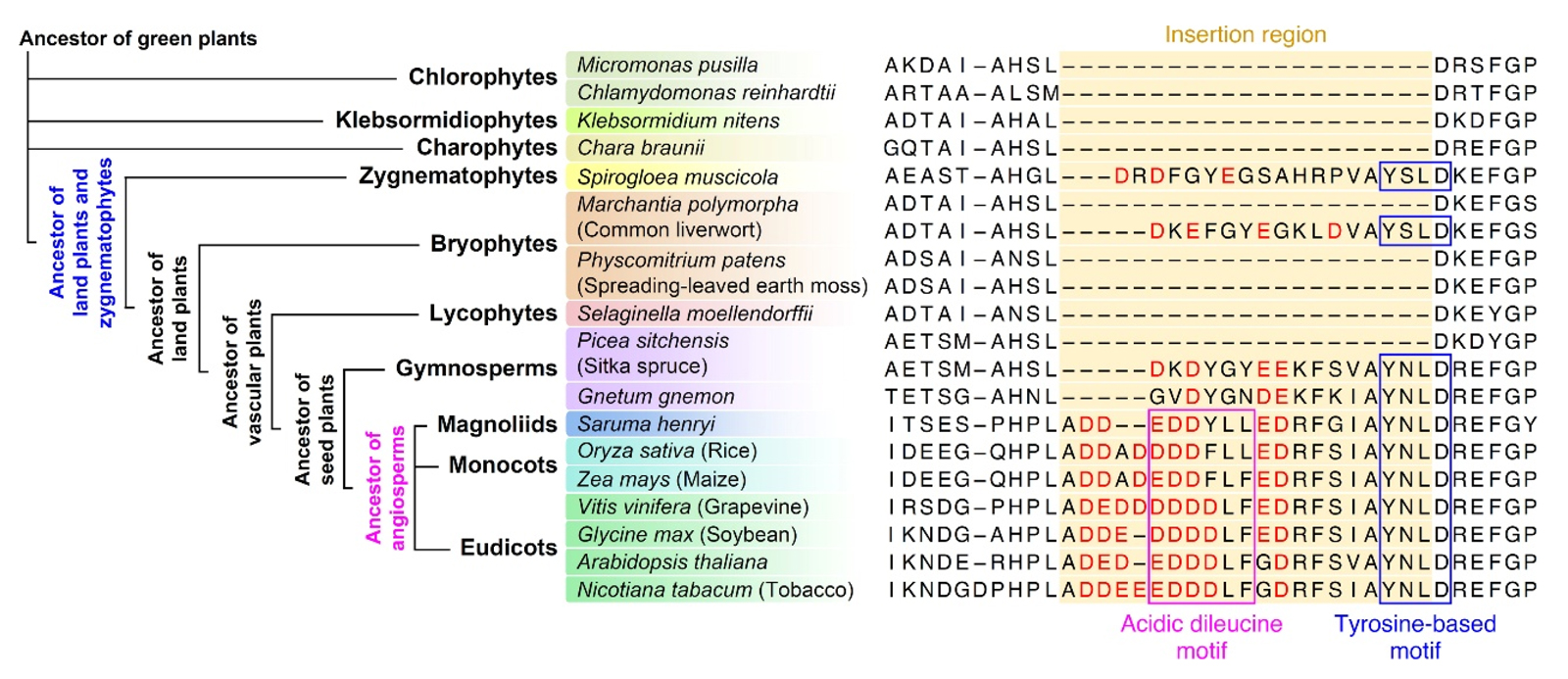 Figure 2. Stepwise Evolution of Insertion Sequences in VAMP72 Proteins Across the Green Plant Lineage
Figure 2. Stepwise Evolution of Insertion Sequences in VAMP72 Proteins Across the Green Plant Lineage
A phylogenetic tree (left) illustrates the major green plant lineages, from chlorophyte algae to angiosperms. Aligned amino acid sequences (right) show the insertion regions in VAMP72 and VAMP727 homologs from representative species. The emergence of key sequence motifs—such as tyrosine-based and acidic dileucine motifs—occurred in a stepwise fashion during evolution, ultimately giving rise to VAMP727.
Created by Takashi Ueda and Masaru Fujimoto.
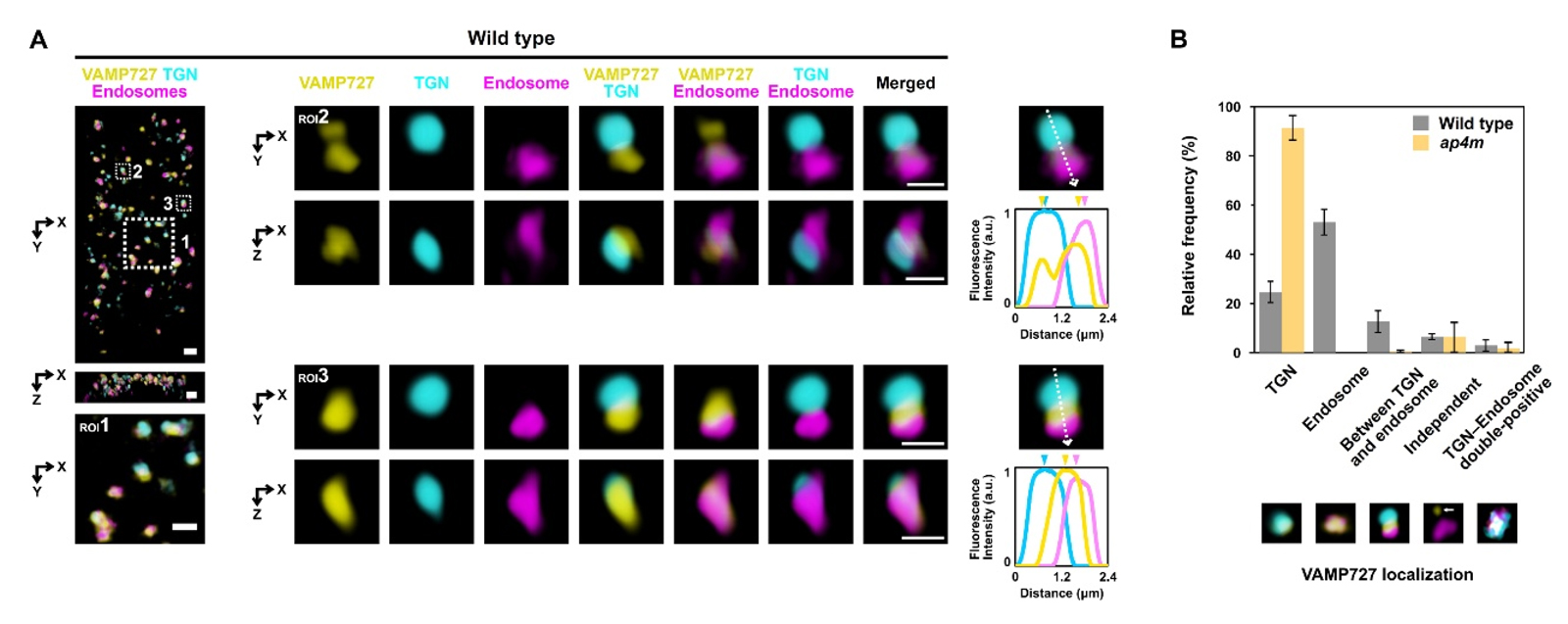 Figure 3. AP-4 complex mediates the sorting of VAMP727 for vacuolar transport at the trans-Golgi network
Figure 3. AP-4 complex mediates the sorting of VAMP727 for vacuolar transport at the trans-Golgi network
Super-resolution confocal live imaging microscopy (SCLIM) reveals the subcellular localization of VAMP727 in wild-type and
ap4m mutant
Arabidopsis root epidermal cells. In wild-type cells (A and B), VAMP727 predominantly localizes to late endosomes, the trans-Golgi network (TGN), and the interface between these compartments. In
ap4m mutants lacking a functional AP-4 complex, VAMP727 exhibits a broadened distribution across the TGN and diminished presence in late endosomes (B). These observations indicate that the AP-4 complex plays a critical role in partitioning VAMP727 within the TGN to facilitate its selective incorporation into the vacuolar transport pathway. Scale bars: 1 µm.
Created by Yutaro Shimizu and Masaru Fujimoto.
[EurekAlert!]
https://www.eurekalert.org/news-releases/1084380
[Original research article]
Current Biology
Neofunctionalization of VAMP7 opened up a plant-unique vacuolar transport pathway
Masaru Fujimoto, Yutaro Shimizu, Yoko Ito, Kazuo Ebine, Naoki Minamino, Takehiko Kanazawa, Yoichiro Fukao, Akihiko Nakano, Tomohiro Uemura, Takashi Ueda
DOI:
https://doi.org/10.1016/j.cub.2025.04.062




 Figure 1. How VAMP72 evolved into VAMP727 through stepwise changes in plants
Figure 1. How VAMP72 evolved into VAMP727 through stepwise changes in plants Figure 2. Stepwise Evolution of Insertion Sequences in VAMP72 Proteins Across the Green Plant Lineage
Figure 2. Stepwise Evolution of Insertion Sequences in VAMP72 Proteins Across the Green Plant Lineage Figure 3. AP-4 complex mediates the sorting of VAMP727 for vacuolar transport at the trans-Golgi network
Figure 3. AP-4 complex mediates the sorting of VAMP727 for vacuolar transport at the trans-Golgi network