
National Institute for Basic Biology



2017.12.08
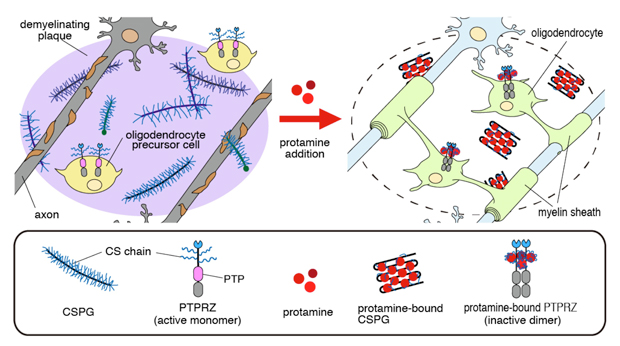
Remyelination is a critical repair process in demyelinating diseases such as multiple sclerosis (MS). Chondroitin sulfate proteoglycans (CSPGs), a family of large molecules consisting of a core protein and glycosaminoglycans comsposed of chains of alternating sugars, are enriched in glial scars. In MS lesions, CSPGs accumulate as constituents of demyelinating plaques, which inhibit the migration and differentiation of oligodendrocyte precursor cells and remyelination.
The research group of Researcher Akihiro Fujikawa and Professor Masaharu Noda of the National Institute for Basic Biology (NIBB) developed a screening method to obtain neutralizing agents for canceling the inhibitory effects of CSPGs on oligodendrocyte differentiation. The group found that the inhibitory activity of a representative extracellular matrix CSPG molecule, aggrecan, was neutralized by a polycationic peptide, protamine, that is clinically used to stop the anticoagulant effect of heparin.
They also found that protamine inhibited a receptor-type protein tyrosine phosphatase, PTPRZ. The extracellular region of PTPRZ is highly modified with CS chains and its phosphatase activity contributes to maintain immature states of oligodendrocyte precursor cells. Intranasal administration of protamine enhanced myelination in the developing mouse brain, and its intraventricular administration significantly improved the remyelination of cuprizone-induced lesions in adult mice.
These results suggest that protamine neutralizes the inhibitory activity of CSPGs, thereby permitting migration of oligodendrocyte precursor cells into demyelinating sites and promoting their differentiation to mature oligodendrocytes for remyelination.
The results of this research were published in ‘PLOS ONE’ on Dec 7, 2017.
###
PLOS ONE
“Protamine neutralizes chondroitin sulfate proteoglycan-mediated inhibition of oligodendrocyte differentiation”
Kazuya Kuboyama, Naomi Tanga, Ryoko Suzuki, Akihiro Fujikawa, and Masaharu Noda