RESEARCH: Difference between animal and plant cells
Assoc. Prof. Takashi Murata in collaboration with Dr. Daisuke Tamaoki in Toyama Univ. and Prof. Tomomi Nemoto in Hokkaido Univ. Graduate students are welcome to join.
The last common ancestor of metazoans and land plants is inferred to have been unicellular. Before multicellularization, cells obtained different characteristics in each lineage, which later reflected on the developmental diversity between the two lineages. For example, cortical microtubules originated in the common unicellular ancestor of charophytes and land plants, and should be an evolutionary constraint for later evolution in these lineages. Therefore, the study of cellular machinery is important to understand the evolution of development as well as cellular machinery itself.
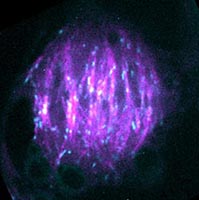
Barrel-shaped mitotic spindle of a tobacco BY-2 cell, showing typical acentrosomal spindles of land plants. Microtubules and their plus ends are labeled with mCherry-tubulin (magenta) and EB1-Citrine (blue), respectively.
(continuing)
In addition to the evolutionary importance of cellular characters, molecular mechanisms at the cellular level remain largely unexplored in land plants, having been mostly studied in unicellular organisms such as yeasts and cultured metazoan cells. For example, the lack of centrosomes in land plant cells is well known, but we do not know how land plant cells can form mitotic spindles, which are formed from centrosomes in metazoan cells.
Microtubule organization during a cell cycle has prominent differences between metazoans and land plants. Associate Professor Takashi Murata and our colleagues first focused on the nucleation mechanism of the cortical microtubules, which are specific in the part of green alga and land plants that regulates the plane of cell division and the direction of cell elongation. When we visualized the microtubule using a tubulin fused with green fluorescent protein (GFP) and performed time-lapse imaging, we found that a cortical microtubule nucleated on a preexisting microtubule, forming a branch with a regular angle of 40 degrees. This was the first clear evidence of branching of microtubules in living organisms (Murata et al. 2005 Nature Cell Biol. 7: 961). Microtubule-dependent microtubule nucleation was contemporarily reported in fission yeast cells, but the angles of nascent microtubules were parallel to the existing microtubules. Microtubule nucleation on existing microtubules of mitotic spindles in mammalian cells was later suggested. Comparisons of molecular mechanisms causing differences in nucleation among the lineages will provide insights into the evolution of microtubule organization, which will be one of our future studies.
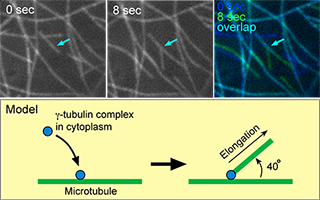
Microtubules in plant cortical arrays nucleate on existing microtubules as branches. Top panels show microtubule nucleation visualized by GFP-alpha-tubulin in living leaf epidermal cells of Arabidopsis thaliana. An arrow in each panel indicates a microtubule nucleation site. Images at 0 (left) and 8 (center) seconds, and merged image (right) showing changes of microtubules in 8 seconds are shown. Bottom panel shows proposed model of microtubule nucleation on existing microtubules.
The cells of land plants and their sister group, charophycean green algae, divide by the insertion of cell plates at cytokinesis. This is in contrast to other green algae, in which the invagination of the plasma membrane separates daughter cells at cytokinesis. The cell plate appears in the middle of daughter nuclei, expands centrifugally towards the cell periphery, and finally fuses to the parental cell wall. Cell wall materials are transported to the expanding cell plate with a phragmoplast, which is mainly composed of microtubules. Centrifugal expansion of the phragmoplast is a driving force for that of the cell plate, although elucidating the molecular mechanism for the expansion was a challenge. We have found that gamma-tubulin complexes on existing phragmoplast microtubules nucleate new microtubules as branches. Although elongation of the branched microtubules is likely a driving force of the phragmoplast expansion, the mechanism by which phragmoplast microtubules redistribute towards the cell periphery is unclear. We found that the phragmoplast array comprises stable microtubule bundles and dynamic microtubules. Furthermore, we found that the dynamic microtubules are nucleated by γ-tubulin on stable bundles. The dynamic microtubules elongate at the plus ends and form new bundles preferentially at the leading edge of the phragmoplast. At the same time, they are moved away from the cell plate, maintaining a restricted distribution of minus ends. We propose that cycles of attachment of γ-tubulin complexes onto the microtubule bundles, microtubule nucleation and bundling, accompanied by minus-end-directed motility, drive the centrifugal development of the phragmoplast (Murata et al. 2013. Nature Communications 4: 1967).
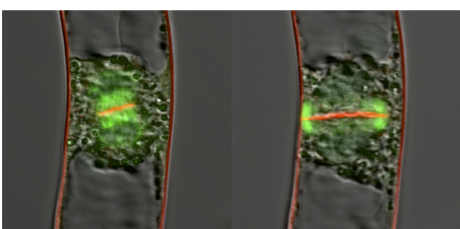
Centrifugal expansion of the phragmoplast. Green, phragmoplast; Red, cell plate and membrane.
At mitosis, all eukaryotic cells divide chromosomes to two daughter cells by a spindle body, which is composed of microtubules. For accurate distribution of the chromosomes, the spindle has two poles. The centrosomes, which act as microtubule organizing centers, ensure formation of the two poles in metazoan cells. In contrast, the cells of land plants and their sister group, charophycean green algae, form the bipolar spindle in the absence of centrosomes. For understanding the mechanism of acentrosomal spindle formation, steps of microtubule reorganization during spindle formation should be visualized. It is challenging, however, to visualize microtubule reorganization during spindle formation in living plant cells, because large numbers of microtubules rapidly redistribute in 3-dimensional spaces. We collaborated with Prof. Tomomi Nemoto in Hokkaido University and developed a two-photon spinning disk confocal microscope, which enables 3-dimensional imaging of living cells with high temporal and spatial resolution. Our data shows that microtubules elongate from template microtubules on the nuclear envelope upon nuclear envelope breakdown. The template microtubules are distinct from the spindle microtubules because they disappear during spindle development. The data suggests that microtubules which had been organized before spindle formation are the organizer of the bipolar spindle.

