Research
NIBB Annual Report
| 2019 | 2018 | 2017 | 2016 | 2015 | 2014 |
| 2013 | 2012 | 2011 | 2010 | 2009 | 2008 |
Our research
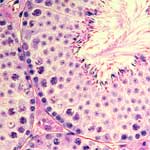 Many animals, including mammalians, produce a large number of sperm over a long period for reliable reproduction.
On the other hand, each sperm correctly replicates and transmits the genetic information to the next generation.
The ultimate goal of the Division of Germ Cell Biology is to reveal how seemingly contradictory, albeit essentially important, productivity and fidelity are both achieved.
To this end, we pay special attention to the sperm stem cells.
Many animals, including mammalians, produce a large number of sperm over a long period for reliable reproduction.
On the other hand, each sperm correctly replicates and transmits the genetic information to the next generation.
The ultimate goal of the Division of Germ Cell Biology is to reveal how seemingly contradictory, albeit essentially important, productivity and fidelity are both achieved.
To this end, we pay special attention to the sperm stem cells.
What are the sperm stem cell?
The continual sperm production is supported by the "sperm stem cells," termed formally "SSCs" after spermatogonial or spermatogenic stem cells.
SSCs maintain a delicate balance between self-renewal and differentiation to sustain life-long spermatogenesis.
It has been a central question which fraction of spermatogonia, the "early stage of cells before meiosis, are SSCs and where and how they behave in the testis.
The backbone of mammalian SSC research was established from the 1950s to the 1970s, relying on morphological analyses of fixed testis specimens.
This led to a proposal of the prevailing stem cell model called the "As model."
This model proposed that singly isolated spermatogonia (A-single or As cells) act as SSCs, while all syncytia are committed to differentiation.
Since then, important breakthroughs and new technologies have made mammalian SSCs one of the most intensively studied tissue stem cells.
These include the establishment of transplantation assay, in vitro long-term culture, and molecular-genetic approaches.
For a fuller understanding of SSCs
Based on these and other preceding studies, our lab has been tackling to the nature of mouse SSCs and their behavior and regulations in vivo, i.e., within the microenvironment of the testicular seminiferous tubules. In particular, we have introduced the scale of time using updated technologies, i.e., intravital live-imaging and pulse-labeling studies, which revealed many different aspects of dynamical behaviors of SSCs. Our lab is also interested in other interesting aspects of germ cell biology. Key methods and achievements made in the lab are listed below (linked with more details).
Research methods
- Intravital live-imaging of labeled cells in the testis
- Pulse-labeling and clonal cell fate analysis
- Biophysical modeling
Stem cell identity and behavior:
- As cells and syncytia together comprise a single SSC pool
- Cells inclined for differentiation revert to SSCs
- SSCs follow variable fate behaviors
- An multistate SSC model
Niche regulation of stem cells:
- SSCs show active motion in an “open niche” associated with the vasculature
- SSC heterogeneity underpins the asymmetric fates of SSCs
- SSCs compete for self-renewal factors in an open niche
- Periodic differentiation of SSCs in the tissue
Stem cell transplantation:
Temperature-sensitivity of spermatogenesis
Research Topics
Intravital live-imaging of labeled cells in the testis
To follow the real-time behaviors of fluorescence-labeled spermatogonia, we developed an intravital live-imaging system keeping mice under anesthesia (Yoshida et al., Science 2007). Using this system, several cell behaviors, including division, motion, shape change, and death of spermatogonia have been filmed for some days. In particular, the fragmentation of spermatogonial syncytia through intracellular bridge breakdown motivated us to revisit the As model that assumes that syncytia do not fragment or contribute to the self-renewing pool (Hara et al., Cell Stem Cell 2014).
Pulse-labeling and clonal cell fate analysis
To complement intravital live-imaging, we also developed a pulse-labeling setting for SSC research. Using tamoxifen-inducible CreER-loxP system, we pulse-labeled fractions of spermatogonia to follow their fate in physiological and homeostatic conditions, over much larger time and space scales. In addition to conventional population-level fate analysis, sparse labeling allows the clone (or single cell-level) fate analyses. We utilize this method in many studies including Nakagawa et al., Dev Cell 2007; Hara et al., Cell Stem Cell 2014; Nakamura et al., Cell Stem Cell; Nakagawa et al., Cell Reports 2021.
Biophysical modeling
The intravital live-imaging and pulse-labeling experiments provide quantitative data about the fate behavior of spermatogonia in vivo at a high (e.g., single-cell) resolution. These data provided insights into the SSC dynamics when combined with statistical, mathematical, and biophysical analyses. We have conducted these theoretical analyses in collaboration with the Lab of Ben Simons, a theoretical physicist and biologist at Cambridge University, leading to many publications (Klein et al., Cell Stem Cell 2010 ; Hara et al., Cell Stem Cell 2014; Kitadate et al., Cell Stem Cell 2019; Nakamura et al., Cell Stem Cell 2021).
As cells and syncytia together comprise a single SSC pool
Using the intravital live-imaging setting, we found that most divisions of As cells are incomplete and give rise to a syncytia (A-pair or Apr), which further form longer syncytia (A-align or Aal). We also found that these syncytia fragment into pieces and supply As cells at considerable frequencies. By combination with pulse-labeling clonal fate analysis and theoretical evaluations, we propose that SSCs reversibly change their morphology between singly-isolated and syncytial states, which together comprise a single self-renewing pool (Nakagawa et al., Science 2010; Hara et al., Cell Stem Cell 2014).
Cells inclined for differentiation can revert to SSCs
Generally for tissue stem cells, it has been widely thought that once stem cells have been destined for differentiation, they never replicate themselves (or self-renew) again. Using pulse-labeling studies, we found that spermatogonia that barely self-renew in homeostasis maintain the stem cell potential up to a particular stage of differentiation. Because they can revert to the undifferentiated state and support long-term self-renewal with high frequencies, particularly during regeneration after tissue damage or after transplantation (Nakagawa et al., Developmental Cell 2007; Nakagawa et al., Science 2010).
SSCs follow variable and stochastic fate behaviors
Stem cells are often thought to invariably undergo asymmetric division, which produces one stem cell and one differentiating cell to maintain homeostasis. However, using clonal fate analyses of pulse-labeling spermatogonia, we found that individual SSCs follow highly variable fates between clones. Still, collectively, they achieve a perfect balance between self-renewal and differentiation at a population level. By mathematical modeling, we found that individual SSCs stochastically (probabilistically, in another word) follow division, differentiation, syncytial fragmentation, and differentiation at particular rates. Such dynamics is called “population asymmetry”, contrary to “division asymmetry” (Klein et al., Cell Stem Cell 2010; Hara et al., Cell Stem Cell 2014).
An multistate SSC model
It is getting apparent recently, due to the rapid progress of single-cell analyses in particular, that SSCs (specifically, GFRα1+ undifferentiated spermatogonia) are still heterogeneous. Following identifying a gene (Plvap) expressed in the primitive-most small fraction, we pulse-labeled the subfractions of GFRα1+ cells. The results suggest that SSCs transit continually and reversibly between self-renewal-biased (Plvap+) and differentiation-primed (Sox3+) states in homeostasis, forming robustly a heterogeneous self-renewing pool. This “multistate stem cell dynamics” has been validated by a biophysical modeling scheme, offering inclusive insights explaining the seemingly contradictory theories discussed in the field (Nakagawa et al., Cell Reports 2021).
SSCs show active motion in an “open niche” associated with the vasculature
In many tissues, stem cells receive self-renewing signals in definitive regions termed “stem cell niche”. However, our intravital live imaging studies revealed that SSCs are scattered between differentiating progeny and highly motile over the basement membrane of the seminiferous tubules, showing preference near blood vessels. Making a stark contrast to the afore-mentioned “closed (or defined) niche,;&rdquo such tissue microenvironment is called an “open (or facultative) niche” ( Yoshida et al., Science 2007; Hara et al., Cell Stem Cell 2014; Yoshida et al., DGD 2018; Yoshida et al., Cold Spring Harbor Perspect Biol. 2020).
SSC heterogeneity underpins the asymmetric fates of SSCs
In an open niche, a fraction of SSCs remain undifferentiated while others differentiate to maintain long-term tissue homeostasis. We found that, in a testicular open niche, asymmetric fates of SSCs are achieved by their differential susceptibility to signaling molecules that are broadly distributed over the seminiferous tubules. In particular, some SSC fractions resist differentiation-promoting Wnt signal by expressing a Wnt-inhibitor, Shias 6 (Tokue et al., Stem Cell Reports 2017); another SSC subset responds to retinoic acid (RA) to differentiate by upregulating an RA receptor, RARγ (Ikami et al., Development 2015).
SSCs compete for self-renewal factors in an open niche
We also questioned the mechanism that maintains a constant SSC density in the open niche of the testis. We found that the SSC density linearly correlates with the dose of FGFs secreted from the testis somatic cells. FGFs promote the SSC self-renewal by being taken up and degraded by SSCs. Combined with biophysical modeling, these findings together suggest that SSC density homeostasis is achieved through competition among SSCs for limited amounts of FGFs. This novel mechanism of tissue stem cell regulation is proposed as the “mitogen competition model” (Kitadate et al., Cell Stem Cell 2019).
Periodic differentiation of SSCs
Strikingly, within the seminiferous tubules, exit from the self-renewing SSC pool and subsequent differentiation to matured sperm occur synchronously every 8.6 days. This is called the seminiferous epithelial cycle. Through modulating the retinoic acid (RA) biosynthesis and detailing the expression of RA metabolism enzymes, we propose that the periodic differentiation is triggered by periodic synthesis and degradation of RA, which also coordinates the differentiation of Sertoli cells (Sugimoto et al., Mech. Dev. 2012).
Clonal analysis and tuning of post-transplantation SSC fate
A prominent function of tissue stem cells is to restore lost tissue structure and function by transplantation. In mouse spermatogenesis, when dispersed SSCs are injected into host animals' seminiferous tubules whose germ cells have been depleted, SSCs engraft and regenerate spermatogenesis (Brinster et al., 1994). However, what happens to donor SSCs and their derivatives during repopulation was largely elusive. We found that donor SSCs follow stochastic fate with significant similarity with homeostasis. We further succeeded in tuning the donor SSC fate to increase the repopulation efficiency, which would promise the practical use of this technology (Nakamura et al., Cell Stem Cell 2021).
Temperature-dependent meiosis failure
Mammalian spermatogenesis is a heat-vulnerable process, with elevated testis temperature leading to spermatogenesis failure. However, the preceding in vivo studies could not fully detail temperature dependence or the underlying events. Collaborating with Takehiko Ogawa Lab at Yokohama City University, we conducted ex vivo testis organ culture at controlled different temperatures. We found that spermatogenesis failed at multiple steps, showing sharp temperature dependencies. In particular, at 38°C (body core temperature), meiotic prophase I is damaged, showing increased DNA double-strand breaks (DSBs) and compromised DSB repair, leading to apoptosis at the meiotic checkpoint. We hope these findings would ignite investigating why mammalian spermatogenesis undergoes at low temperatures (Hirano et al., Commun. Biol., 2022).