National Insitute for Basic Biology

National Insitute for Basic Biology

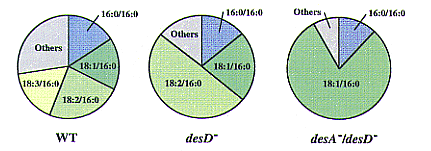
|
| Figure.1 Changes in composition of lipid molecular species by step-wise depletion of desaturases in Synechocystis sp. PCC 6803. WT, wild-type strain; desD -, a mutant of A6 desaturase in which the desD gene was disrupted by insertion of a chloramphenicol resistance gene cartridge; desA -/desD -, double mutant of Æ6 and Æ12 desaturases in which the desA gene in the desD - mutant was mutated by insertion of a kanamycin resistance gene cartridge. |
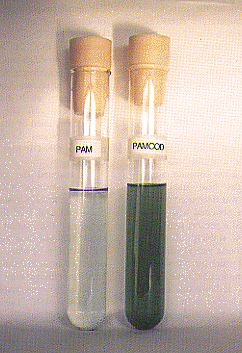
|
| Figure 2. Growth under saline conditions in control cells (PAM) and transformed cells (PAMCOD) in which the codA gene (for choline oxidase) was introduced in the chromosome of Synechococcus sp. PCC 7942 and was overexpressed under control of the ConII promoter. Cells were grown at 30°C for 6 days in a medium containing O.4 M NaCl. |