National Insitute for Basic Biology

National Insitute for Basic Biology

|
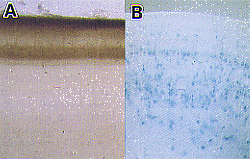
| Fig. 1 A) Lamina-specific projection of retinal axons in the chick optic tectum. The anterogradely-labeled retinal terminals (brown) make synapses in some restricted laminae. B) Recombinant adenovirus-mediated gene transfer into the chick optic tectum. Adenovirus carrying lacZ (E. coli ß-galactosidase) was injected into the mesencephalic ventricle. After one week, many cells in various laminae express the introduced gene (blue). |