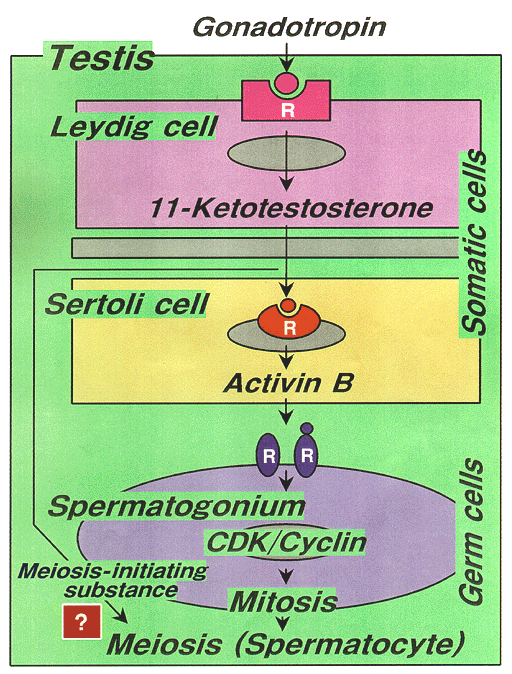
NATIONAL INSITUTE FOR BASIC BIOLOGY

National Institute for Basic Biology
DIVISION OF REPRODUCTIVE BIOLOGY
- Professor:
- Yoshitaka Nagahama
- Associate Professor:
- Michiyasu Yoshikuni
- Research Associates:
- Minoru Tanaka
- Tohru Kobayashi
- Institute Research Fellow:
- Akihiko Yamaguchi
- JSPS Postdoctoral Fellows:
- Mika Tokumoto
- Yoshinao Katsu
- Daisuke Kobayashi
- Craig Morrey
- Catherine Dreanno
- Balasubramarian Senthilkumaran
- Yoshinao Katsu
- JSPS Research Associates:
- Yuichi Ohba
- Yasutoshi Yoshiura
- Masaru Matsuda
- Toshitaka Ikeuchi
- Yasutoshi Yoshiura
- Graduate Students:
- Masatada Watanabe (Graduate University for Advanced Studies)
- Gui-Jin Guan (Graduate University for Advanced Studies)
- Ryo Horiuchi (Graduate University for Advanced Studies)
- Gui-Jin Guan (Graduate University for Advanced Studies)
- Monbusho Foreign Scientist:
- Allen Schuetz (Johns Hopkins University)
- Srinivas K. Saidapur (Karnatak University)
- Visiting Scientist: Graham Young (University of Otago)
- Srinivas K. Saidapur (Karnatak University)