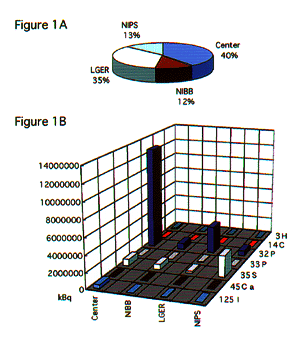
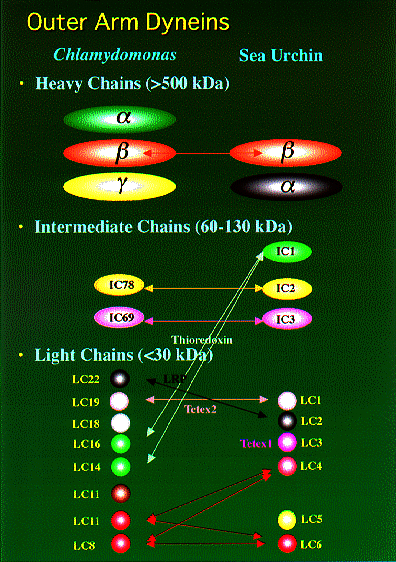
NATIONAL INSITUTE FOR BASIC BIOLOGY

National Institute for Basic Biology
RADIOISOTOPE FACILITY
(Managed by NIBB)
- Head:
- Masaharu Noda
- Associate Professor:
- Kazuo Ogawa
- Technical Staffs:
- Kazuhiko Furukawa (Radiation
- Protection Supervisor),
- Yosuke Kato (Radiation Protection Supervisor),
- Yoshimi Matsuda (Radiation Protection Supervisor)
- Protection Supervisor),
- Supporting Staff:
- Takayo Ito, Risako Shirai