Fig. 2.
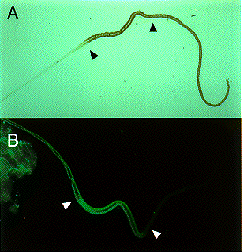
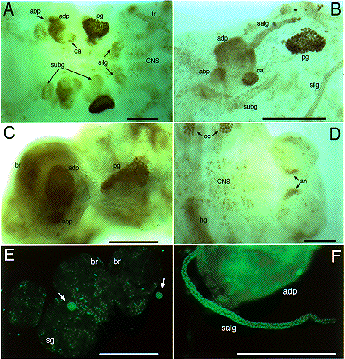
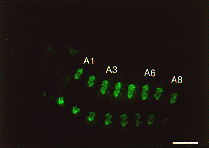
Expression of SGF-3/POU-M1 in embryos. (A) At stage 20, signals are seen in precursor cells of the prothoracic glands (pg), adductor plates (adp), abductor plates (abp), silk glands (silg), subbuccal glands (subg), corpora allata (ca), tracheal system (tr), and in some cells of the central nervous system (CNS). (B) At stage 21, the salivary glands are elongated from the posterior part of the adp. Both invaginated cells in the anterior mandibular and posterior maxillary segments are fused to form the subg. (C) At stage 23, signals are also seen in the adp, abp, and the pg that reveal mature form. (D) Magnified in posterior region of an embryo at stage 21. Signals are seen in parts of the anus. (E) At stage 25, signals are seen in some cells of the brain (br) and the suboesophageal ganglion (sg), and in the ca (arrows). (F) At stage 25, signals are seen in the salivary glands (salg) and the adp. Anterior side is always to the left and dorsal side is up. All scale bars represent 100 mm. Revised from Kokubo et al. (1997) Dev. Genes Evol. 206, in press.
|
NATIONAL INSITUTE FOR BASIC BIOLOGY