Research
Research
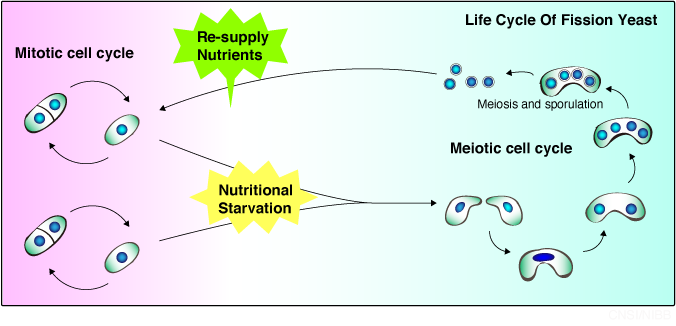
In our laboratory we use the fission yeast Schizosaccharomyces pombe, the simplest organism that performs meiosis,to research the mechanism by which cells switch from mitosis,
the kind of cell division that divides cells equally to create two identical cells, to meiosis,
which is essential for bringing forth genetically diverse progeny.
Signaling pathways that regulate the onset of sexual differentiation
We have been trying to elucidate how fission yeast cells switch their mode of cell cycle from mitotic to meiotic.
We focus on a highly conserved kinase, namely Target of rapamycin (TOR) kinase,
which plays key roles in the recognition of nutrition and the onset of sexual differentiation in fission yeast.
The molecular mechanisms that establish the meiosis-specific transcription profile
Expression of hundreds of genes is upregulated during meiosis. We have shown that specific control of the stability of meiotic transcripts,
which is orchestrated by the interplay between RNA-binding proteins and a long non-coding RNA,
contributes to the meiosis-specific gene expression in fission yeast.
Understanding precise mechanisms of this control will shed light on the regulation of timely gene expression during meiosis.