National Insitute for Basic Biology

National Insitute for Basic Biology

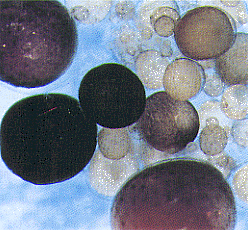
|
| Fig.1 Whole mount in situhybridization showing P450 aromatase mRNA in medaka ovarian follicles. |
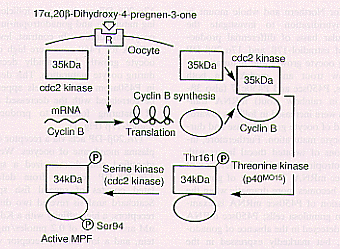
|
| Fig.2 Molecular mechanisms of 17 a ,20 B -dihydroxy-4-pregnen-3one induced MPF activation in goldfish oocytes. |