National Insitute for Basic Biology

National Insitute for Basic Biology

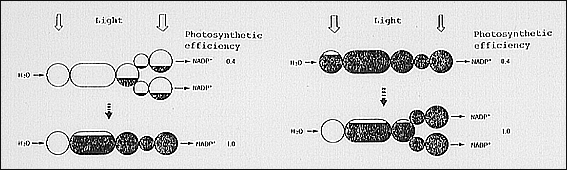
|
| Fig. 1. Schematic presentation of regulation of PSI/PSII stoichiometry and electron transport state. Shaded area in each circle indicates level of reduced state of each component. Reduced PSII and oxidized PSI are photochemically inactive, respectively, and large occurrence of such states causes low efficiency of photo-synthesis. PQ, plastoquinone; Cyt b 6 - f, cytochrome b 6 - f complex; PC, plastocyanin. |