4.7 Miniprotoplast
preparation for BAC library
Naomi Sumikawa and
Takashi Murata
Materials:
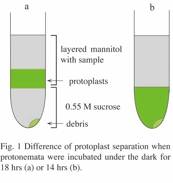
Protonemata were
cultured on BCDAT medium layered with cellophane for 6 days under continuous
light after inoculation, and then they were kept in the dark for 18-24 hrs. The dark incubation
removes starch grains from chloroplasts. Do not use BCDTAG medium, which
contains glucose, because glucose in the medium prevents removal of starch. It is very important
to keep more than 18 hours in the dark in order to isolate protoplast from
debris (see Fig. 1).
Preparation:
Solutions and tubes
needed
1. Digestive solution:
2% driselase and 10%
mannitol in H2O (160 ml for 4
tubes) at room temperature
2. 10% mannitol in H20
(kept in ice-water)
3. MP solution 50
ml
PS/HEPES (pH 7.3) 18
ml
0.6
M Sorbitol 30 ml
1
M MgCl2 2 ml
Freshly
prepare MP solution for each experiment.
PS/HEPES (pH 7.3)
Percoll
(Amersham) 1 L
sucrose 250
g
Dissolve
and adjust pH to 7.3 with HEPES powder.
Store
at 4C
3. 35 ml polycarbonate cfg tubes (kept in ice-water or at
4C°).
4. One 50 ml
polypropylene tube containing 10 ml 0.55 M sucrose in H2O. Keep in ice-water.
5. It is preferable to
keep all plastic, glass dishes, and solutions except the digestive solution and
the MP solution
at 4C. This may not be seriously necessary.
Keep the digestive
solution and the MP solution at room temperature.
6. Use wide-pore
round-tip pipettes kept at 4C°.
Protocol
0. Chill cfg and swing rotors
(for 35 ml and 50 ml) at 2C°
1. Harvest eighty 9-cm petri dishes of
protonemata layered with cellophane.
You can get 6.4 x 10^7
miniprotoplasts. If you need, you can reduce the scale.
2. Move protonemata from 20 dishes to a 35 ml round-bottom
polycarbonate cfg tube (PC tube) containing 40 ml digestive solution [2%
driselase and 10% mannitol in H2O]. Four tubes for 80
dishes.
3. Incubate for 40 min at 25℃. Gently but
thoroughly rotate tubes once in 10 min.
4. Filtrate through a
nylon seat with 50 µm mesh to new four PC tubes. You can use a nylon seat
for four tubes. If flow-through becomes worse, wash the seat with 10% mannitol.
5. Store for 5 min at room
temperature for complete digestion.
6. cfg. 1,000 rpm /
170 x g, swing rotor, 2 min at 2C°
7. Discard sup with
wide-pore pipette (NO Decantation) and
keep on ice.
8. Gently add 5 ml
ice-cold 10% mannitol to each tube and gently suspend with wide and round-pore pipette on ice.
9. Add 25 ml of ice-cold 10% mannitol
and gently mix.
10. cfg. 1,000 rpm /
170 x g, swing rotor, 2 min at 2C°
11. Repeat 7, 8
12. Gently layer 5 ml
x 4 suspended solution on 0.55 M sucrose solution in a ice-cold 50 ml
polypropylene tube.
13. cfg. 2,000 rpm /
670 x g, swing rotor, 10 min at 2C°
14. Collect middle
green zone (Fig. 1a) with cold pipette and gently pour to two new 35 ml PC
tubes on ice.
15. Slowly add 30 ml ice-cold 10%
mannitol with gentle mixing
on ice.
16. cfg. 1,000 rpm /
170 x g, swing rotor, 2 min at 2C°
17. Discard sup with
wide-pore pipet (NO Decantation) and keep at room temperature.
18. Gently add 25 ml
MP solution and gently and thoroughly mix.
19. cfg. 15,000 rpm /
25,000 x g, angle
rotor, 45 min at 20C°
20. Chill a 35 ml PC tube on
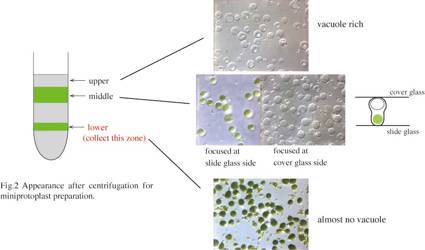
ice.
20. Move a lower zone
(Fig. 2) to the ice-cold PC tube.
21. Slowly add 30 ml ice-cold
10% mannitol with gentle mixing.
22. cfg. 1,000 rpm /
170 x g, swing rotor, 2 min at 2C°
23. Discard sup with a wide-pore pipette (NO Decantation).
24. Add an appropriate
solution to suspend ppt for following experiments.