
 |
LABORATORY OF TRESS RESPONSE |
| Associate Professor: | MIKAMI, Koji |
|
The aim of this laboratory is to understand molecular mechanisms of the flexibility in modification of the cell polarity during morphogenesis in plants under the changes in environmental conditions. As their sessile nature, growth and development of plants are largely influenced by environmental factors, thus plants must percept and response to these factors to regenerate the growth axis. In the responses to environmental stimuli, phosphoinositide-specific phospholipase C (PI-PLC) plays the essential role in the generation of two second messengers, inositol-1,4,5-trisphosphate and diacylglycerol through the hydrolysis of phosphatidylinositol-4,5-bisphospate (PIP2). Plant PI-PLC is now thought to be involved in responses to gravity and light, both of which effect the modification of the cell polarity by generation of a physiological asymmetry. In moss plants, such as Physcomitrella patens and Ceratodonpurpureus, changes in cytosolic Ca2+ concentration in caulonemal cells are associated with the generation of buds, the precursors of gametophores, which is regulated by blue light and cytokinin. In addition, the red-light-induced phototropic responses of protonemal tip cells are mediated by phytochrome. It has been shown that red-light-irradiation leads to an increase of the PI-PLC activity and the phototropic bending follows the production of a dynamic Ca2+ gradient in the protonemal tip cells. Moreover, Physcomitrella is now recognized as a model system for plants with easy application of molecular genetic approaches such as gene-targeted mutagenesis via the homologous recombination. Therefore, it is reasonable to employ the moss Physcomitrella for genetical and molecular investigations of the roles of plant PI-PLCs in the modification of cell polarity by environmental factors. |
I. Structural characteristics of Physcomitrella PI-PLCsSearching of the plant EST databases with BLAST algorithm against AtPLC1S from Arabidiopsis thalianarevealed four Physcomitrella cDNA clones, PPU141114, PPU140521, PPU070504 and PPU161218. For the first three ESTs derived from the same mRNA, the 5’-race and the subsequent 3’-race reactions resulted in the isolation of a corresponding full-length cDNA of 2,424 bp containing an ORF for a PI-PLC homologue of 633 amino acids and calculated molecular mass of 70.8 kDa, which was designated PpPLC1. In addition, a 2,423 bp full-length cDNA corresponding PPU161218 was isolated by the 5’-race reaction, which contains an ORF for a PI-PLC homologue of 639 amino acid and calculated molecular mass of 71.8 kDa, designated PpPLC2. The overall structure of PpPLC1 and PpPLC2 was similar to those of known plant PI-PLCs comprising the catalytic domain and the C2 domain. PI-PLC isoforms in mammals have beendivided into four classes, β-, γ-, δ- and ε-types, all of which contain the catalytic domain that consists of X and Y regions and regulatory domains such as pleckstrin homology (PH), EF-hand and C2 domains. In contrast, plant PI-PLCs analyzed so far all show δ-type organization but lacking the PH and typical EF-hand domains. The N domain, which is conserved in Arabidopsis PLCs as a regulatory domain with structural similarity to the second loop of the EF-hand domain of rat PLCδ1, was found in the N-terminal extensions in PpPLC1 andPpPLC2, although there was an insertion in the N domain of PpPLC2. Since the PH and EF-hand domains are responsible for membrane-localization and Ca2+-binding in mammals the mode of activation of plant PI-PLCs is probably different from those in mammals. |
II. Physcomitrella has typical and novel types of PI-PLCsThe in vitroactivity of recombinant His-tagged PpPLC1 and His-tagged PpPLC2 was examined. His-tagged PpPLC1 hydrolyzed PIP2 with maximum activity of 38 nmol/min/mg of protein at a physiological concentration of Ca2+ around 10 µM and lower activity at higher Ca2+ concentrations, consistent with other recombinant plant PLCs. In contrast, His-tagged PpPLC2 hydrolyzed PIP2 with very low activity under the same conditions. When phosphatidylinositol (PI) was used as a substrate in the reactions with various concentrations of Ca2+, both His-tagged PpPLC1 and His-tagged PpPLC2 showed a very low-hydrolyzing activity of 1.3 and 9.4 nmol/min/mg of protein, respectively, at 10 m µM Ca2+. However, at 1 mM Ca2+, His-tagged PpPLC2 hydrolyzed PI very efficiently (22 nmol/min/mg of protein) than His-tagged PpPLC1 (3.5 nmol/min/mg of protein). Together with their structural characteristics, I conclude that PpPLC1 and PpPLC2 are typical and novel types of plant PLC, respectively. In the X and Y regions, 11 amino acids have been identified as essential residues for mammalian PI-PLC activity via binding to Ca2+ and substrate. I found that all of them are well conserved in PpPLC1, although a conserved serine residue was replaced by asparagine residue at amino acid position 409 in PpPLC2. In fact, PLC-like proteins with amino acid substitutions of residues important for PLC activity have already been isolated from rat and human, which have no PIP2-dependent PLC activity. Thus, it seems that the loss of PIP2-dependent activity in PpPLC2 may be due to amino acid substitution in the catalytic domain and/or its abnormal N domain. |
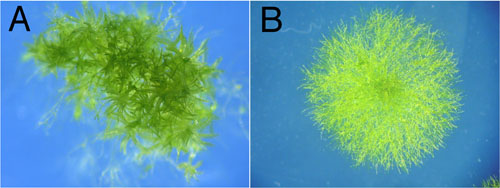
III. PpPLC1 is involved in cytokinin and gravity responsesTo elucidate the physiological functions of PpPLC1, targeted knockout mutants of the PpPLC1 gene were generated via homologous recombination. To construct a plasmid for gene targeting, a 35S-promotor-driven neomycin phosphotransferase II (nptII) cassette providing G418 resistance was inserted into a 3509 bp fragment of the PpPLC1 genomic clone. After transfomation of Physcomitrella protoplasts with the targeting construct, G418-resistance transgenic lines were obtained and screened by PCR to confirm the specific disruption of the PpPLC1 gene. Protonemal filaments grown under standard conditions showed a clear difference between wild type (WT) and plc1, the mutants failing to form gametophores (Fig. 1). The formation of gametophores in Physcomitrella follows two independent developmental steps. First, an immature side-branch is induced from an intercalary caulonemal cell, which was shown to be regulated by blue light (BL) through cryptochromesSecondly, the side-branch initial differentiates into a gametophore bud. This is known to be a cytokinin-dependent step in Physcomitrella. By checking the response of protonemal cells to BL and cytokinin, it was concluded that the absence of gametophores in plc1 is due to a defect in cytokinin signaling. |
|
|
Fig. 1. Comparison of visible phenotypes between WT and plc1 knockout. Leafy gametophores, which were well produced in WT (A), were not observed in plc1 |
|
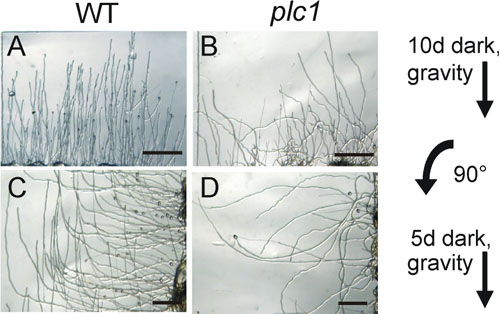
In addition, the protonemal filaments of plc1 have a strongly reduced ability to response to the gravity (Fig. 2). WT plants produce uniformly negative-gravitropically growing protonemal filaments prior to unilateral red light irradiation, when protonemal filaments were cultured on vertical agar plates in the dark (Fig. 2A)However, the protonemal filaments of plc1 have a strongly reduced ability to grow negatively gravitropically in the dark (Fig. 2B). Moreover, after rotating the cultures 90°, WT filaments responded with a subsequent change in the growth direction, according to the gravitropic stimuli, whereas the plc1 continued to grow randomly (Figs. 2C and 2D). Similar results were obtained after growth in diffuse white light (100 µmol m-2 s-1) for two weeks in a vertical orientation. Therefore, PpPLC1 plays an important role in the detection of and/or response to gravitropic stimuli in Physcomitrella In summary, PpPLC1 has diverse functions including the formation of gametophores from side branches in protonemal filaments and the response to gravity in apical tip cells. To understand molecular mechanisms of modification of the cell polarity, it is necessary to elucidate how plants percept cytokinin and gravity, how these signals are transduced in the cells and how PpPLC1 acts to modify the cell polarity. Whereas most of the knowledge about the physiological function of plant PI-PLC is based on inhibitor studies that might cause non-specific effects, Physcomitrella constitutes a most attractive experimental system for studying these points at the molecular level, which based on the efficient targeted gene disruption via homologous recombination to generate plc1 null-mutants. Current works are focused on the mode of activation of PpPLC1 to elucidate how environmental stimuli lead the modification of the cell polarity in plants. |
|
|
Fig. 2.Comparison of gravitropic response of protonemal filaments between WT and plc1 knockout. Protonemal filaments of WT (A, C) and plc1 (B, D) were cultured on vertical agar surfaces for ten days in the dark (A, B). Cultures were rotated after ten days for 90° and further kept in darkness for additional 5 days (C, D). Scale bars represent 500 µm. |
Publication list: |
Original articles |
|
Mikami, K., Repp, A., Graebe-Abts, E. and Hartmann, E. (2004) Isolation of cDNAs encoding typical and novel types of phosphoinositide-specific phospholipase C from the moss Physcomitrella patens. J. Exp. Bot. 55, 1437-1439. |
|
Repp, A., Mikami, K., Mittmann, F. and Hartmann, E. (2004) Phosphoinositide-specific phospholipase C is involved in cytokinin and gravity responses in the moss Physcomitrella patens. Plant J. 40, 250-259. |
Review article |
|
Mikami, K. and Hartmann, E. (2004) Lipid metabolism in mosses. In, New Frontiers in Bryology: Physiology, Molecular Biology and Functional Genomics (A.J. Wood, M.J. Oliver and D.J. Cove, eds.), Kluwer Academic Publishers, Dordrecht, The Netherlands, pp. 133-155. |
