
 |
LABORATORY OF HOTOENVIRONMENTAL BIOLOGY |
| Professor (Adjunct): | WATANABE, Masakatsu (Sep. 1 -) |
| Associate Professor: | WATANABE, Masakatsu (- Aug. 31) |
| Postdoctoral Fellows: | ISEKI, Mineo 1) |
| YOSHIKAWA, Shinya 2) | |
| SUZUKI, Takeshi 2) | |
| Visiting Scientist: | ITO, Shinji |
| Technical Assistants: | KOUMURA, Yoshiko 2) |
| ITO, Makiko | |
| 1)PRESTO, JST (Group Leader) | |
| 2)PRESTO, JST |
|
Photoreceptive and signal transduction mechanisms of phototaxis of unicellular algae are studied action spectroscopically (Watanabe 2004) by measuring computerized-videomicrographs of the motile behavior of the cells at the cellular and subcellular levels. Photoreceptive and signal transduction mechanisms of algal gene expression were also studied by action spectroscopy (Kräbs et al. 2004). A novel blue-light receptor with an effector role was found from Euglena gracilis (Fig. 1; Iseki et al. 2002, Nature 415, 1047-1051): Euglena gracilis, a unicellular flagellate, shows blue-light type photomovements.The action spectra indicate the involvement of flavoproteins as the photoreceptors mediating them.The paraflagellar body (PFB), a swelling near the base of the flagellum has been considered as a photosensing organelle for the photomovements. To identify the photoreceptors in the PFB, we isolated PFBs and purified the flavoproteins therein. The purified flavoprotein (ca. 400 kDa), with noncovalently bound FAD, seemed to be a heterotetramer of α- and β-subunits. Predicted amino acid sequences of each of the subunits were similar to each other and contained two FAD-binding domains each followed by an adenylyl cyclase catalytic domain. The flavoprotein showed an adenylyl cyclase activity, which was elevated by blue-light irradiation. Thus, the flavoprotein (PAC, photoactivated adenylyl cyclase) can directly transduce a light signal into a change in the intracellular cyclic AMP level without any other signal transduction proteins. |
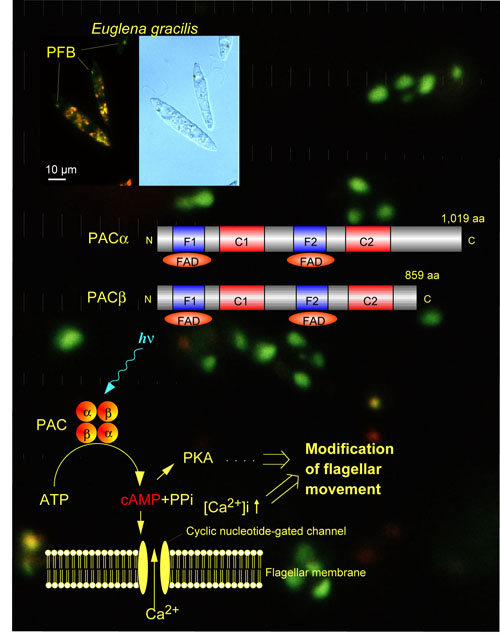
|
|
Fig.1 Schematic diagram showing domain structures of PAC subunits and working hypothesis of action mechanism of PAC to photocontrol Euglena flagellar movement. |
|
The involvement of PAC in positive and negative phototaxis (steering response with respect to stimulus light direction) was also demonstrated by its knock-down using RNAi through collaboration with Professor D.-P. Häder’s laboratory of Phillips University, Erlangen. Germany (Fig. 2; Ntefidou et al. 2003 Plant Physiol. 133, 1517-1521.). |
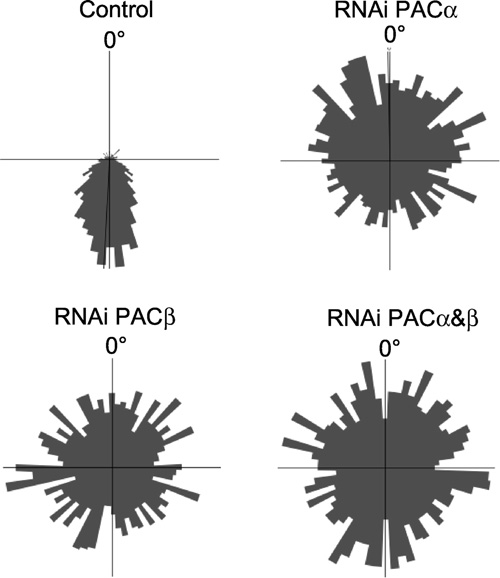
|
|
Fig.2 Swimming tracks of Euglena cells summarized in circular histograms under unilateral light irradiation. Control cells swimming with high precision away from the light source and RNAi-treated cells showing random swimming. (From Ntefidou et al. 2003, Plant Physiol. 133, 1517-1521) |
|
To gain an insight into the evolution of this unique protein, similar sequences were searched for in several euglenoids by RT-PCR using degenerate primers. Two similar transcripts were detected in each of the four phototrophic euglenoids, Euglena stellata, Colacium sideropus, Eutreptia viridis, Eutreptiella gymnastica, and in an osmotrophic (i. e., obtaining nutrients by absorption) one, Khawkinea quartana, but not in a phagotrophic euglenoid, Petalomonas cantuscygni. Each of them seemed to be orthologous to PAC&aipha; and PACβ, respectively, and had the same domain structure as PAC subunits each of which is composed of two flavin binding domains, F1 and F2, each followed by an adenylyl cyclase catalytic domain, C1 and C2, respectively. This fact implies that they constitute a functional photoactivated adenylyl cyclase like PAC.Phylogenetic analysis of the adenylyl cyclase catalytic domains revealed that they belong to a bacterial cluster, not to a trypanosomal one. In addition, two trypanosome-type adenylyl cyclases were discovered in E. gracilisIn contrast to PAC, deduced amino acid sequences of the trypanosome-type adenylyl cyclases indicated that they are integral membrane proteins with a membrane spanning region at the midpoint of them, followed by an adenylyl cyclase catalytic domain which seems cytoplasmic. Overall, we propose that PAC might have been transferred to euglenoids on the occasion of secondary endosymbiosis (Fig. 3; Koumura et al. 2004). |
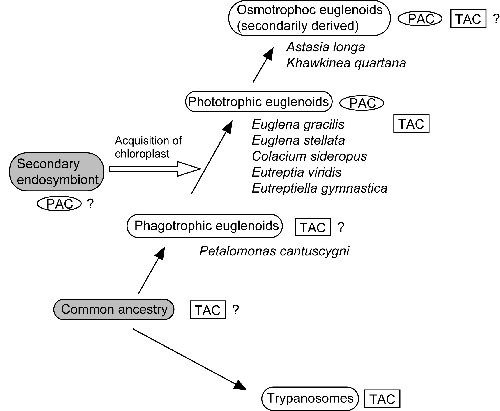
|
|
Fig.3 Working hypothesis on the origin of PAC. Presence of orthologs of PAC subunits (‘PAC’ in an oval) and trypanosome-type adanylyl cyclases (‘TAC’ in a rectangle) are indicated on the well accepted evolutionary history of euglenoids. (From Koumura et al. 2004). |
|
Basic kinetic studies on photoactivation of PAC in vitro and in vivo were done (Yoshikawa et al. submitted): in vitro photoactivation by blue-light showed reciprocity between fluence rate and duration of irradiation in the fluence rate range of 2-50 µmol/m2/s. Intermittent irradiation caused activation of PAC in a photon fluence dependent manner irrespective of cycle periods. The time course of the change in intracellular cAMP level agreed well with that of the step-up photophobic response. Overall, an increase in intracellular cAMP level evoked by photoactivation of PAC is a key event of the step-up photophobic response. |
Publication list: |
|
Koumura, Y., Suzuki, T., Yoshikawa, S., Watanabe, M. and Iseki, M. (2004) The origin of photoactivated adenylyl cyclase (PAC), the Euglena blue-light receptor: phylogenetic analysisi of orthologues of PAC subunits from several euglenoids and trypanosome-type adenylyl cyclases from Euglena gracilis. Photochem. Photobiol. Sci., 3, 580-586. |
|
Kräbs, G., Watanabe, M. and Wiencke, C. (2004) A monochromatic action spectrum for the photoinduction of the UV-absorbing mycosporine-like amino acid shinorine in the red alga Chondrus crispus. Photochem. Photobiol. 79, 515-519. |
|
Watanabe, M. (2004) Action Spectroscopy for Photosensory Processes. In CRC Handbook of Organic Photochemistry and Photobiology, 2nd edition, (Horspool, W. and Lenci, F. eds), CRC Press, Boca Raton, pp. 115.1-115.16. |
