
 |
DIVISION OF MOLECULAR |
ENVIRONMENTAL ENDOCRINOLOGY |
| Professor: | IGUCHI, Taisen |
| Associate Professor: | WATANABE, Hajime |
| Research Associate: | KATSU, Yoshinao |
| Technical Staff: | MIZUTANI, Takeshi |
| NIBB Research Fellow: | URUSHITANI, Hiroshi |
| Postdoctoral Fellow: | SONE, Kiyoaki 1) (- Oct. ’04) |
| Graduate Students: | MIYAGAWA, Shinichi |
| NAGATA, Emiko | |
| KOBAYASHI, Mika | |
| ISHIMOTO, Yoichi | |
| Visiting Scientist: | KATO, Hideo 2) |
| Technical Assistants: | SUZUKI, Atsuko 1) (- Oct. ’04) |
| KOBAYASHI, Kaoru | |
| TAKAHASHI, Eri | |
| HINAGO, Megumi | |
| ICHIKAWA, Rie 1) (- Oct. ’04) | |
| GOTO, Mayu | |
| Secretary: | IMAIZUMI, Taeko |
| 1)CREST, JST | |
| 2)from Nihon Bioresearch Inc. |
|
Synthetic chemicals found in the environment have the capacity to disrupt endocrine system development and function in both wildlife and humans. This has drawn public concern since many of these chemicals may bind to estrogen receptors (ER) and evoke estrogenic effects. Early evidence that estrogenic chemicals could pose a threat to human health during development came from studies of diethylstilbestrol (DES), which was used to prevent premature birth and spontaneous abortion.Laboratory experiments have demonstrated that exposure of animals to sex hormones during perinatal life can cause permanent and irreversible alterations of the endocrine and reproductive systems as well as the immune system, nervous system, bone, muscle, and liver in both sexes. Although many of these chemicals may bind to ER and evoke estrogenic effects in wildlife and humans, the effects of estrogen are not well understood even now. Thus, understanding the effects of sex hormones at the molecular level, especially during development, is very important to resolve these problems. |
|
|
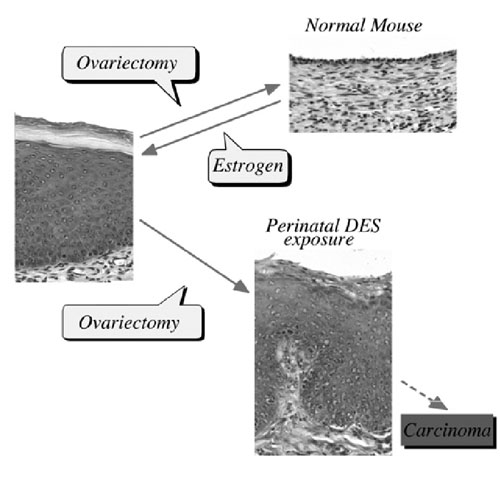
FIG. 1 Scheme of estrogen-dependent and -independent vaginal epithelial cells in mice induced by neonatal estrogenization. |
I. Estrogen-induced irreversible changesPerinatal sex-hormone exposure has been found to induce lesions in reproductive tracts in female mice. The possible relevance of the mouse findings to thedevelopment of cancer in humans has been emphasized. In the early seventies, a close correlation between occurrence of vaginal clear cell adenocarcinoma in young women and early intrauterine exposure to DES was demonstrated. Many chemicals released into the environment have the potential to disrupt endocrine function in wildlife and humans. Some of these chemicals induce estrogenic activity by binding to ER.The neonatal mouse model has been utilized especially to demonstrate the long-term effects of early sex hormone exposure on the female reproductive tract. Neonatal treatment of female mice with estrogens induces various abnormalities of the reproductive tract: ovary-independent cervicovaginal keratinization, adenosis, uterine hypoplasia, epithelial metaplasia, oviductal tumors, polyovular follicles (PF) and polyfollicular ovaries. Female reproductive tracts in mice exposed prenatally to estrogen show altered expression of Hoxa genes and Wnt genes and the analysis of knockout mice lacking Hoxa-10 or Wnt7a show uterine hypoplasia. The growth response of neonatally DES-exposed reproductive organs to estrogen is reduced, as are ER levels and epidermal growth factor (EGF) receptor levels, in addition to other hormone receptor levels. |
|
|
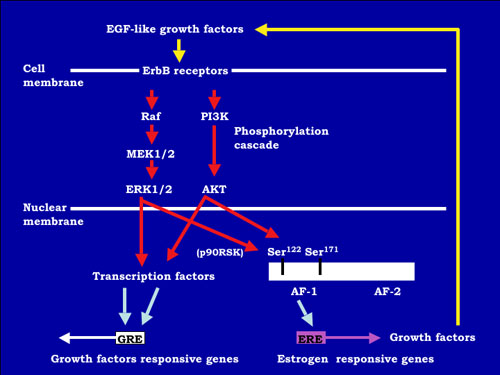
FIG.2 A hypothetical model for the estrogen-independent ER activation pathway in mouse vagina. EGF-like growth factors activate the protein-phosphorylation cascade via erbB receptors. In nuclear, estrogen receptor is phosphorylated on serine 122 and 171 in AF-1 domain. Furthermore, transcription factors are activated by phosphorylation. These phosphorylations induce the transcriptional activity of ER, and then growth factors are expressed via estrogen-response element (ERE). Growth factors induced by ER activate EGF-receptors. |
|
Growth factors and ER signaling cooperate to play essential roles in cell proliferation, differentiation and tumor progression in mouse reproductive organs. The mechanisms of the estrogen-dependent and -independent pathways remain unknown. EGFR and erbB2 were activated in the vaginal epithelium of mice by estrogen treatment. This activation was also encountered in vaginae from neonatally DES-exposed mice, along with the expression of EGF, TGF-α, HB-EGF, amphiregulin and neuregulin. Immunochitochemical analysis indicated that erbB2 was primarily expressed in vaginal epithelium. Serine 118 and 167 located in the AF-1 domain of ER a were phosphorylated in these vaginae. AG825, AG1478 or ICI 182,780, that are erbB2, EGFR and ER antagonists, respectively, administration blocked proliferation of vaginal epithelium induced by neonatal DES exposure. Signal transduction via EGFR and erbB2 could be related to the estrogen-induced vaginal changes and persistent erbBs phosphorylation and sustained expression of EGF-like growth factors, leading to ERα activation that may result in cancerous lesions in vaginae from neonatally DES- exposed mice later in life. To identify estrogen-responsive genes related to the proliferation and differentiation of mouse vaginal epithelial cells, we used differential display and identified a novel c-type lectin that encodes a membrane protein with a c-type lectin domain in the carboxyl-terminal region. Characterization of mRNA expression indicates that estrogen regulates the gene encodeing this novel c-type lectin in mouse vagina. Furthermore, this c-type lectin is found in epithelial cells, but not stromal cells, suggesting that it may be an important factor in the stratification and/or cornification of the vaginal epithelium of mice. We are continuing efforts to analyze its function during proliferation and differentiation in mouse vagina by estrogen treatment. Estrogenic compounds such as bisphenol A (BPA) and nonylphenol as well as dioxins and PCBs were found in the human umbilical cord.BPA can easily cross the placenta and enter the fetus in Japanese monkey and mice. BPA can be found in fetal brain, testis and uterus when given to pregnant mice and monkeys. Neonatal exposure to a high BPA dose induced ovary-independent vaginal changes, polyovular follciles and infertility lacking corpora lutera. Prenatal exposure to a low BPA dose induced acceleration of vaginal opening in the offspring. Thus, the developing mammal is sensitive to exposure to estrogenic agents. |
|
|
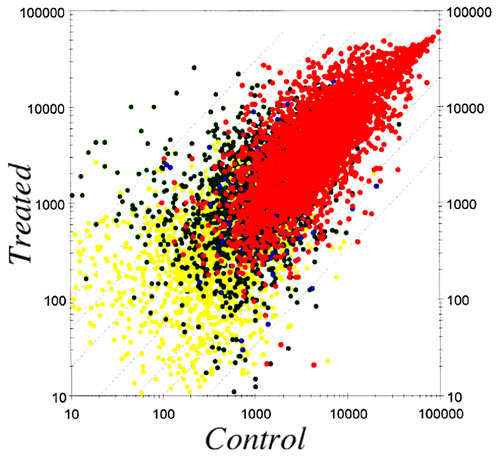
FIG.3 Scatter plot of average expression levels in control and chemical-treated uterus |
II. MicroArray analysisIn order to clarify the molecular mechanisms of these effects, we are studying changes in gene expression patterns induced by perinatal exposure to chemicals or estrogen using differential display and DNA microarray techiniques. We have found genes possibly related to the ovary-independent changes by differential display. We also have clustered groups of genes that are responsive to estrogenic stimuli in uterus by using the DNA microarray system. We need to understand the molecular background of the critical period during development, the low dose effect of estrogenic chemicals and the molecular metabolism of hormones and hormone-like agents in animals including humans. |
III. Effect of estrogen on reptile, amphibian and fishes |
|
|
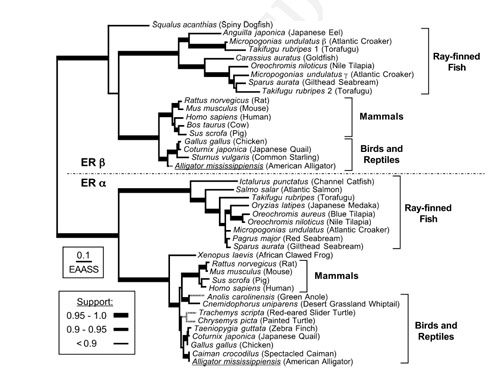
FIG. 4 Evolutionary relationships of estrogen receptor sequences. |
|
During embryogenesis, exogenous estrogen exposure induces abnormal sex differentiation and the abnormal bone formation in African clawed frog, Xenopus laevis, the cyprinodont fish, mummichog (Fundulus heteroclitus), mosquitofish (Gambusia affinis). To analyze the function of estrogen, we have isolated cDNA clones of ER αand β from F. heteroclitus, G. affinis. In UK rivers exposure of roach (Rutilus rutilus – a common cyprinid fish) to effluents from sewage treatment works, containing complex mitures of endocrine disrupting chemicals (EDCs) has been shown to alter sexual development and impact negatively on their reproductive capabilities. To unravel the mechanisms of disruption of sexual development in roach exposed to EDCs, we have isolated the cDNAs related to sex determination and sex differentiation containing estrogen receptor, aromatase, StAR, Sox9, vasa etc. We are examining the gene expression during gonadal differentiation with or without EDCs exposure. Furthermore, we have isolated cDNAs of steroid hormone receptors from American alligators (Alligator mississippiensis), the red belly turtle (Pseudemys nelsoni) and Japanese giant salamander (Andrias japanicus). As the estrogen-responsive genes must play important roles, we are isolating the estrogen-responsive genes to understand the molecular physiology of estrogen action. Japanese tree frog (Hyla japonica) takes water through ventral skin. We found that sex steroids and endocrine disruptors interfere with water absorption through ventral skin in frogs. Further, using the amphibian and fish as model animals we aim to analyze the effects of numerous chemicals released into the environment on endocrine system function in wildlife. |
IV. Molecular Target SearchAbnormalities caused by endocrine disrupting chemicals are reported but the molecular mechanisms of the effects are not well studied. Although estrogen receptor is one of the strongest candidates possibly responsible for the endocrine disrupting function of many chemicals, it alone cannot explain the variety of phenomena induced by endocrine disrupting chemicals. Thus, we are also looking for new target molecules that may be responsible for endocrine disruption.In parallel, we also are studying the ligand-binding mechanisms of nuclear receptors to hormones and other chemicals. |
Publication list: |
|
Adachi, T., Ono, Y., Koh, K.B., Takashima, K., Tainaka, H., Matsuno, Y., Nakagawa, S., Todaka, E., Sakurai, K., Fukata, H., Iguchi, T., Komiyama, M. and Mori, C. (2004) Long-term alteration of gene expression without morphological change in testis after neonatal exposure to genistein in mice: toxicogenomic analysis using cDNA microarray. Food Chem. Toxicol. 42, 445-452. |
|
Kato, H., Iwata, T., Katsu, Y., Watanabe, H., Ohta, Y. and Iguchi, T. (2004) Evaluation of estrogenic activity in diets for experimental animals using in vitro assay. J. Agric. Food Chem 52, 1410-1414. |
|
Katsu, Y., Bermudez, D.S., Braun, E., Helbing, C., Miyagawa, S., Gunderson, M.P., Kohno, S., Bryan, T.A., Guillette, L.J. and Iguchi, T. (2004) Molecular cloning of the estrogen and progesterone receptors of the American alligator. Gen. Comp. Endocrinol. 136, 122-133 |
|
Kohno, S., Fujime, M., Kamishima, Y. and Iguchi, T. (2004) Sexually dimorphic basal water absorption at the isolated pelvic patch of Japanese tree frog, Hyla japonica. J. Exp. Zoolog. A Comp. Exp. Biol. 301, 428-438. |
|
Matsuno, Y., Adachi, T., Koh, K.B., Fukata, H., Sugimura, A., Sakurai, K., Shibayama, T., Iguchi, T., Komiyama, M. and Mori, C. (2004) Effect of neonatal exposure to diethylstilbestrol on testicular gene expression in adult mouse: comprehensive analysis with cDNA subtraction method. Int. J. Androl. 27, 115-122. |
|
Miyagawa, S., Katsu, Y., Watanabe, H. and Iguchi T. (2004) Estrogen-independent activation of erbBs signaling and estrogen receptor alpha in the mouse vagina exposed neonatally to diethylstilbestrol. Oncogne 23, 340-349. |
|
Miyagawa, S., Suzuki, A., Katsu, Y., Kobayashi, M., Goto, M., Handa, H., Watanabe, H. and Iguchi, T. (2004) Persistent gene expression in mouse vagina exposed neonatally to diethylstilbestrol. J. Mol. Endocrinol. 32, 663-677. |
|
Okada, A., Ohta, Y., Brody, S.L. and Iguchi, T. (2004) Epithelial c-jun and c-fos are temporally and spatially regulated by estradiol during neonatal rat oviduct differentiation. J. Endocrinol. 182, 219-227. |
|
Okada, A., Ohta, Y., Brody, S.L., Watanabe, H., Krust, A., Chambon, P. and Iguchi, T. (2004) Role of foxj1 and estrogen receptor alpha in ciliated epithelial cell differentiation of the neonatal oviduct. J. Mol. Endocrinol. 32, 615-625. |
|
Sato, T., Fukazawa, Y., Ohta, Y. and Iguchi, T. (2004) Involvement of growth factors in induction of persistent proliferation of vaginal epithelium of mice exposed neonatally to diethylstilbestrol. Reprod. Toxicol. 19, 43-51. |
|
Seiwa, C., Nakahara, J., Komiyama, T., Katsu, Y., Iguchi, T. and Asou, H. (2004) Bisphenol A exerts thyroid-hormone-like effects on mouse oligodendrocyte precursor cells. Neuroendocrinology 80, 21-30. |
|
Sone, K., Hinago, M., Kitayama, A., Morokuma, J., Ueno, N., Watanabe, H. and Iguchi, T. (2004) Effects of 17beta-estradiol, nonylphenol, and bisphenol-A on developing Xenopus laevis embryos. Gen. Comp. Endocrinol. 138, 228-236. |
|
Uchida, D., Yamashita, M., Kitano, T. and Iguchi, T. (2004) An aromatase inhibitor or high water temperature induce oocyte apoptosis and depletion of P450 aromatase activity in the gonads ofgenetic female zebrafish during sex-reversal. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 137, 11-20. |
|
Watanebe, H., Suzuki, A., Goto, M., Lubahn, D.B., Handa, H. and Iguchi, T. (2004) Tissue-specific estrogenic and non-estrogenic effects of a xenoestrogen, nonylphenol. J. Mol. Endocrinol. 33, 243-252. |
|
Watanabe, H., Suzuki, A., Goto, M., Ohsako, S., Tohyama, C., Handa, H. and Iguchi, T. (2004) Comparative uterine gene expression analysis after dioxin and estradiol administration. J. Mol. Endocrinol. 33, 763-771. |
|
Yoshinaga, N., Shiraishi, E., Yamamoto, T., Iguchi, T., Abe, S. and Kitano, T. (2004) Sexually dimorphic expression of a teleost homologue of Mullerian inhibiting substance during gonadal sex differentiation in Japanese flounder, Paralichthys olivaceus. Biochem. Biophys. Res. Commun. 322, 508-513. |
