

|
DIVISION OF CELLULAR REGULATION |
| Professor: | MURATA, Norio |
| Research Associate: | SUZUKI, Iwane |
| JSPS Research Fellow: | SHIVAJI, S 1) |
| JSPS Postdoctoral Fellows: | JOGADHENU, S. S. Prakash 1) |
| TAKAHASHI, Shunichi | |
| Postdoctoral Fellows: | ALLAKHVERDIEV, Suleyman I. 2) |
| KANESAKI, Yu 3) | |
| Visiting Scientists: | PANICHKIN, Vladimir 4) |
| PAITHOONRANGSARID, Kalyanee 5) | |
| LOS, Dmitry A. 6) | |
| SLABAS, Antoni R. 7) | |
| PING, Xang 7) | |
| Secretary: | KANESAKI, Kazuko |
|
1)the Centre for Cellular and Molecular Biology,Hyderabad, India 2)the Institute of Basic Biological Problems, Pushchino, Russia 3)Ehime University, Matsuyama, Ehime
4)Moscow State University, Moscow, Russia 5)National Center for Genetic Engineering and Biotechnology, Bangkok, Thailand 6)Plant Physiology Institute, Moscow, Russia 7)University of Durham, Durham, England |
|
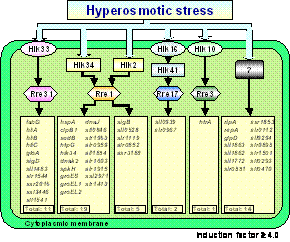
The major thrust of our research efforts is directed towards the comprehensive understanding of the molecular mechanisms that governs the responses of plants and microorganisms to new environments. In particular, our attention is focused on the perception and transduction of various stress signals, such as extreme temperatures, osmosis and salinity. Another line of our research is focused on the molecular mechanisms of photodamage and repair of the photosynthetic machinery under severe stress conditions. In 2004, significant progress was made in the following areas using the cyanobacterium, Synechocystis sp. PCC 6803 (hereafter Synechocystis). I. Crosstalk of signal transduction in the two-component systems.Cells perceive the hyperosmotic signal and transduce it to regulate the expression of a large number of genes. Two-component systems that consist of a histidine kinase (Hik) and a response regulator (Rre) are widely distributed from bacteria to higher plants as the mechanism for intracellular signal transduction. The Synechocystis genome encodes 47 Hiks and 45 Rres. To identify Hiks and Rres that are involved in the perception and transduction of hyperosmotic signals, we screened knockout libraries of Hiks and Rres by RNA slot-blot and genome-wide DNA microarray analyses. We identified four two-component systems, Hik33-Rre31, Hik34-Rre1, Hik16-Hik41-Rre17 and Hik10-Rre3 and an additional potential two-component system, Hik2-Rre1. Interestingly, Rre1 can perceive the hyperosmotic signals from both Hik34 and Hik2. These results suggest that the crosstalk of hyperosmotic signals occurs in Synechocystis cells (Figure 1). Hyperosmotic stress probably induces several phenomena such as rigidification of membranes, decreases in cytoplasmic volume, and changes in hydration state of intracellular proteins. It seems likely that Hik34 and Hik2 might perceive such kinds of stimulus and transduce the signals to Rre1 to regulate the expression of a number of genes. Genes whose expression was regulated by Rre1 include a number of important genes, such as chaperons, proteases and a sigma factor. Therefore, it is understandable that cells have multiple pathways to activate Rre1-dependent transcriptional regulation to synthesize proteins, which are indispensable for the acclimation to hyperosmotic stress. These results uncover a part of complicated mechanisms of signal transduction via crosstalk of multiple pathways of two-component systems. |
|
|
Fig. 1. A hypothetical scheme for the two-component signal transduction pathways that are activated in response to hyperosmotic stress and the genes whose expression is regulated in the respective pathways. |
II. The membrane rigidification regulates the cold-inducible gene expression.Changes in the ambient temperature affect the physical properties of biological membranes. To obtain insights into the role of membranes in the mechanism of cold signal perception, we used a mutant of Synechocystis, in which the desA gene for the Δ12 desaturase and the desD gene for the Δ6 desaturase were both inactive as a result of targeted mutagenesis. Cells of the desA-/desD- mutant synthesize only a saturated C16 fatty acid and a monounsaturated C18 fatty acid regardless of the growth temperature, whereas wild-type cells synthesize di-, tri- and tetra-unsaturated C18 fatty acids in addition to the monounsaturated C18 and saturated C16 fatty acids [Tasaka et al. (1996) EMBO J. 15, 6416-6425]. Fourier transform infrared spectrometry revealed that the desA-/desD- mutation rigidified the plasma membrane at physiological temperatures. We applied DNA microarray technique to examine effects of the membrane rigidification on the induction of gene expression upon cold shock. The results demonstrated that the cold inducibility of a part of cold-inducible genes, such as the crh gene for an RNA helicase and the rbp1 gene for an RNA-binding protein, was unaffected by the rigidification. However, the expression of the other cold-inducible genes, such as hliA, hliB and slr1544, was enhanced by membrane rigidification. Moreover, the expression of certain heat-shock genes, namely, the hspA, htpG and dnaK2 genes, was markedly enhanced by the membrane rigidification. We further inactivated the genes for Hik33 and Hik34, which had been identified as sensors of various signals and found that the mutation of these Hiks eliminated the membrane rigidification-dependent enhancement of expression of hli genes and heat-shock genes, respectively. These findings suggest that Hik33 and Hik34 perceive the change in membrane rigidification as an initial signal of the downward shift in temperature to regulate the cold-inducible gene expression. III. Genome-based systematic analysis of Ser/Thr protein kinases and prediction of their function in signal transduction.In addition to Hiks, cyanobacteria normally possess Ser/Thr protein kinases which may function as sensors and/or transducers of environmental signals. The complete genome sequence of Synechocystis harbors 12 putative genes for Ser/Thr protein kinases; seven of the 12 genes encode proteins that belong to a PKN2 subfamily, while the other five to an ABC1 subfamily of Ser/Thr protein kinases. These genes are termed in series as spkA, spkB, spkC, spkD, spkE, spkF and spkG for kinases of PKN2 type and spkH, spkI, spkJ, spkK and spkL for kinases of ABC1 type. The role of these genes for Ser/Thr protein kinases in Synechocystis remained largely unexplored. We systematically mutated all of these genes for Ser/Thr kinase by insertion of antibiotic-resistance gene cassettes and investigated the impact of these mutations on the genome-wide expression of genes by means of DNA microarray analysis. Mutation of seven of the genes, namely, spkA, spkC, spkD, spkG, spkH, spkJ and spkL, markedly changed the gene expression with increases in the expression of some genes or with decreases in the expression of some other genes, whereas mutation of the other five spk genes did not significantly affect the profile of gene expression. These observations predict that at least seven Ser/Thr protein kinases are involved specifically in the regulation of gene expression possibly via a signal transduction pathway. The stress inducibility of genes whose expression was affected by the spk mutations predicts the signal transduction pathway to which the individual Ser/Thr kinases belong. IV. RNA helicase is required for cold acclimation in Synechocystis.DNA microarray analysis revealed that cold stress induced the expression of a large number of genes. The crhR gene for RNA helicase was one of such highly inducible genes. We have observed the following characteristics of the crhR gene and its product CrhR (RNA helicase) of Synechocystis. (1) The crhR gene was highly induced, not only by cold, but also by hyperosmotic and salt stress. (2) The crhR mutant cells exhibited a phenotype of slow growth at low temperatures indicating an important function of CrhR under cold stress conditions. (3) DNA microarray and Northern blotting analyses indicated that the crhR mutation reduced the expression of genes for molecular chaperonins, groES, groEL1 and groEL2. (4) CrhR regulated the level of mRNA of groESL1 and groEL2 by stabilizing the mRNA. (5) Further, we demonstrated that the crhR mutation decreased the GroEL levels during cold stress. (6) The crhR mutation downregulated the expression of groESL1 and groEL2 genes under salt and hyperosmotic stress conditions. In micro-organisms such as E. coli and Synechocystis, the activity of translation is reduced when they are subjected to cold stress. During cold acclimation, several cold shock proteins are synthesized and association of these cold shock proteins with ribosomes resumes the translation. The cold-induced synthesis of GroESL might be important for the translation machinery to maintain the activity at a proper level under cold stress conditions. Thus the cold-induced expression of the crhR gene is very important for Synechocystis cells to acclimate cold stress. V. Photodamage to photosystem II occurs in two-step mechanisms.Light is necessary for photosynthesis but it also damages the photosynthetic machinery, in particular, photosystem II. The current hypothesis for the mechanism of photodamage to photosystem II postulates that excess light energy, which is absorbed by photosynthetic pigments but not utilized in photosynthesis, produces reactive oxygen species that damage the photochemical reaction center of photosystem II. Using monochromatic light generated by the Okazaki Large Spectrograph, we demonstrated that UV or blue light first inactivates the oxygen-evolving complex of photosystem II and then blue or red light, which is absorbed by photosynthetic pigments, inactivates the photochemical reaction center as the second step. These results lead us to conclude that the current hypothesis is not the case and that the photodamage to photosystem II occurs in two steps (Figure 2). The first step is the photodamage to the oxygen-evolving complex of photosystem II probably at the Mn-cluster and its protein environment. The second step is the photodamage to the photochemical reaction center II after the oxygen-evolving complex is inactivated. |
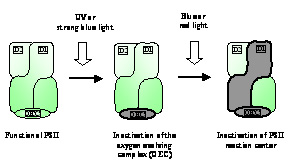 |
Fig. 2. Schematic model for the two-step mechanism of photodamage to photosystem II. |
VI. Environmental factors do not accelerate the photodamage to photosystem II, but inhibit the repair of photosystem IILight inactivates photosystem II, and this phenomenon is referred to as the “photoinhibition”. The extent of photoinhibition is a result of a balance between photodamage to and repair of photosystem II. We developed a system to monitor the photodamage and repair separately and examined the effect of various environmental stresses on photodamage and repair. In contrary to the current concept that environmental stress accelerates photodamage to photosystem II, we clearly demonstrated that environmental stresses do not damage photosystem II directly, but they inhibit the repair of photodamaged photosystem II at the level of translation of the psbA genes which encode the D1 protein, an important component of the photosystem II reaction center. Furthermore, we found in Chlamydomonas cells that the repair of photosystem II was inhibited when the Calvin cycle was inhibited. List of publication:(1) Original articlesS. Suzuki, A Ferjani, I. Suzuki and N. Murata (2004) The SphS-SphR two-component system is the exclusive sensor for the induction of gene expression in response to phosphate limitation in Synechocystis. J. Biol. Chem. 279: 13234-13240 K. Saeki, K. Matsumoto, M. Kinoshita, I. Suzuki, Y. Tasaka, K. Kano, Y. Taguchi, K. Mikami, M. Hirabayashi, N. Kashiwazaki, Y. Hosoi, N. Murata and A. Iritani (2004) Functional expression of a Δ-12 fatty acid desaturase gene from spinach in transgenic pigs. Proc. Natl. Acad. Sci. USA 101: 6361-6366 S. I. Allakhverdiev and N. Murata (2004) Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of photosystem II in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta (Bioenergetics) 1657: 23-32 A. Sakamoto, R. Sulpice, T. Kaneseki, C.-X. Hou, M. Kinoshita, S. Higashi, B. Y. Moon, H. Nonaka and N. Murata (2004) Genetic modification of fatty acid unsatulation of chloroplastic phosphatidylglycerol alters the sensitivity to cold stress. Plant, Cell & Environ., 27: 99-105 Y. Nishiyama, S. I. Allakhverdiev, H. Yamamoto, H. Hayashi and N. Murata (2004) Singlet oxygen inhibits the repair of photosystem II by suppressing the elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry 43: 11321-11330 K. Marin, Y. Kanesaki, D. A. Los, N. Murata, I. Suzuki and M. Hagemann (2004) Gene expression profiling reflects physiological processes in salt acclimation in Synechocystis sp. strain PCC 6803. Plant Physiol. 136: 1-11 E.-J. Park, Z. Jekni, A. Sakamoto, J. DeNoma, R. Yuwansiri, N. Murata and T. H. H. Chen (2004) Genetic engineering of glycinebetaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. Plant J. 40: 474-487 K. Paithoonrangsarid, M. Shoumskaya, Y. Kanesaki, S. Satoh, S. Tabata, D. A. Los, V. Zinchenko, H. Hayashi, M. Tanticharoen, I. Suzuki and N. Murata (2004) Five histidine kinases perceive osmotic stress and regulate distinct sets of genes in Synechocystis. J. Biol. Chem.279: 53078-53086 S. I. Allakhverdiev, Y. Nishiyama, S, Takahashi, S. Miyairi, I. Suzuki and N. Murata (2005) Systematic analyses of the relation of electron transport and ATP synthesis to regulate the photodamage and repair of photosystem II in Synechocystis. Plant Physiol, in press A. Shapiguzov, A. A. Lyukevich, S. I. Allakhverdiev, T. V. Sergeyenko, I. Suzuki, N. Murata and D. A. Los (2005) Osmotic shrinkage of cells of Synechocystis sp. PCC 6803 by water efflux via aquaporins regulates the osmostress-inducible gene expression. Microbiology 151: in press (2) Review articlesD. A. Los and N. Murata (2004) Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta (Biomembrane) 1666:142-157 Y. Nishiyama, S.I. Allakhverdiev and N. Murata (2005) Regulation by environmental conditions of the repair of photosystem II in cyanobacteria. Photoprotection, Photoinhibition, Gene Regulation, and Environment (eds., B. Demming-Adams, W.W. Adams and A.K. Mattoo), Kluwar Academic Publishers Y. Nishiyama, S. I. Allakhverdiev and N. Murata (2005) Inhibition of the repair of Photosystem II by oxidative stress in cyanobacteria. Photosynth. Res., in press |
 |