
 |
DIVISION OF GENOME DYNAMICS |
| Professor: | HORIUCHI‚ Takeshi |
| Research Associates: | KOBAYASHI‚ Takehiko |
| JOHZUKA‚ Katsuki | |
| Technical Staff: | MOROOKA‚ Naoki |
| JSPS Research Fellow: | AUSTEN RD Ganley |
| Postdoctoral Fellows: | KODAMA‚ Ken-ichi |
| OHSUMI‚ Katufumi | |
| SERIZAWA‚ Naomi | |
| Visiting Postdoctoral Fellow: | CUI ‚ Tailing (Apr. 1 -) |
| Visiting Scientist: | UJIIE‚ Yoshifumi |
| Graduate Students: | WATANABE‚ Takaaki |
|
The genomes of higher organisms contain significant amounts of repetitive sequences which, in general, are unstable. At present, neither the physiological function(s) of repeated sequences nor the mechanism controlling instability are fully understood.To clarify these aspects, we are pursuing the following themes usingE. coli and S. cerevisiae: (1) the amplification mechanism of repeated sequences or genes, especially the rRNA repeated genes, (2) the mechanism of replication fork block-dependent recombination, a key reaction that increases or decreases the copy number of rRNA genes, and (3) development of in vivo artificial gene amplification systems. In 2004, work on the following four subjects has advanced our knowledge of the dynamics of the genome. |
I. Transcription-mediated hyper-recombination in HOT1, a recombinational hotspot in S. cerevisiaeRecombination hotspots are DNA sequences which enhance recombination around that region. HOT1 is one of the best-studied mitotic hotspots in yeast. HOT1 consists of two elements; I and E. They are two dis-continuous regions in an rDNA unit. The I-element corresponds to a RNA polymerase I (PolI) transcription promoter which is responsible for 35S ribosomal rRNA gene (rDNA) transcription. The E-element overlaps the enhancer for PolI transcription, containing a replication fork barrier site (FRB) where Fob1 protein, required for fork blocking at RFB, binds specifically. HOT1 stimulates recombination when inserted at novel locations in the genome. For example, when HOT1 is integrated into one of the repeated his4 genes, the fragment enhances recombination between the repeats ~ 100 times. In a PolI defective mutant the HOT1 hotspot activity is abolished, therefore transcription of HOT1 is thought to be an important factor for the recombination stimulation. However, it is not clear whether the transcription itself or other pleiotropic phenotypes stimulate recombination. To investigate the role of transcription, we made a highly activated Pol I transcription system in HOT1 by using a strain whose rDNA repeats are totally deleted (rdnΔΔ). In the rdnΔΔ strain, HOT1 transcription was increased about 14 times compared to wild-type. Recombination activity stimulated by HOT1 in this strain was also elevated, about 15 times, compared to wild-type. These results indicate that the level of Pol I transcription in HOT1 determines efficiency of the recombination. Moreover, Foblp, which is essntial for both the recombination stimulation activity and transcription of HOT1 , was dispensable in the rdnΔΔ strains. This suggests that Fob1p is functioning as a PolI transcriptional activator in the wild-type strain. |
II. SIR2 regulates recombination between different rDNA repeats, but not recombination within individual rRNA genes in yeast.In most eukaryotic organisms, the rRNA genes (rDNA) are clustered in long tandem repeats on one or a few chromosomes. Although the total number of these chromosomal rDNA repeats appears to be maintained at a level appropriate for each organism, genes with such a repeated structure are in general thought to be unstable because of a high frequency of recombinational events. Thus, it might be expected that organisms have developed systems to regulate recombination within rDNA repeats. In the yeast S. cerevisiae, approximately 150 copies of rDNA are maintained on chromosome XII. Recombinational events within the rDNA repeats in normal growing yeast cells appear to be mostly mediated by a FOB1-dependent system. FOB1 is the gene required for fork blocking activity at RFB site, recombination in the rDNA region, and expansion/contraction of rDNA repeats. The latter two activities are likely to be triggered by double-strand breaks at the RFB site and repair of the breaks via gene conversion. On the other hand, the SIR2 gene plays an important role in decreasing the frequency of recombination in yeast rDNA. Sir2p is a protein required for transcriptional silencing at three yeast chromosomal regions, silent mating type loci, telomeres, and rDNA. It is generally believed that Sir2p, perhaps through its NAD+-dependent histone deacetylase activity, plays an essential role in forming a higher order of repressive chromatin structure – heterochromatin - which prevents general access of the PolII machinery and some other macromolecules, thus causing silencing as well as decreasing recombination in the chromosomal rDNA repeats. Therefore, mutations in gene SIR2 increase recombination within rDNA repeats as assayed by marker loss or extrachromosomal rDNA circle formation. We examined the mechanism involved in the increased frequency of recombination in rDNA repeats that is observed in mutants defective in SIR2 functions. We measured the frequency of FOB1-dependent arrest of replication forks, consequent DNA double-strand breaks, and formation of DNA molecules with Holliday junction structures, and found no significant difference between sir2Δ and SIR2 strains. Formal genetic experiments measuring mitotic recombination rates within individual rRNA genes also showed no significant difference between these two strains. Instead, we found a significant decrease in the association of the cohesin subunit Mcd1p (Scc1p) to the rDNA in sir2Δ relative to SIR2 strains. From these and other experiments, we conclude that SIR2prevents unequal sister-chromatid recombination, probably by forming special cohesin structures, without significant effects on recombinational events within individual rRNA genes. |
III. A novel gene amplification system in yeast based on double rolling-circle replication.Gene amplification is involved in various biological phenomena such as cancer development and drug resistance. However, the mechanism is largely unknown because of the complexity of the amplification process. We developed a gene amplification system in Saccharomyces cerevisiae that is based on double rolling-circle replication (DRCR), utilizing break-induced replication (BIR), and this is depicted in the Figure below. This system produced three types of amplification products. Type-1 products contain 5-7 inverted copies of the amplification marker, leu2d. Type-2 products contain 13 to ~100 copies of leu2d (up to ~730 kb increase) with novel arrangement present as randomly oriented sequences flanked by inverted leu2d copies. Type-3 products are acentric multi-copy mini-chromosomes carrying leu2d. Structures of type-2 and -3 products resemble those of homogeneously staining region (HSR) and double minutes (DMs) of higher eukaryotes, respectively. Interestingly, products analogous to these were generated at low frequency without deliberate DNA cleavage. These features strongly suggest that the processes described here may contribute to natural gene amplification in higher eukaryotes. |
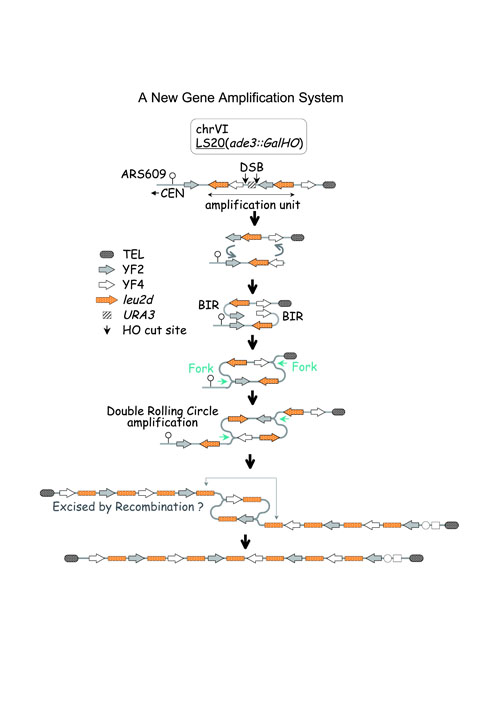
|
|
Figure: A new gene ampilification system in which amplification throuth double rolling circle replication (DRCR) is triggered by break-induced replication (BIR). Top shows the structure of the right terminus region of chromosome V1 where an amplification unit (-leu2d-YF4-HO-URA3-HO-YF2-ieu2d-) was inserted. Induction of double stand breaks (DSBs:shown by the two arrows) by HO endonuclease triggers BIR, which initiates DRCR. An amplification selective marker, leu2d, is used to select amplified clones. The DRCR process is expected to terminate by recombination between lue2d, genes on each bidirectionally elongated arm. ARS: autonomously replicating sequence; CEN: centromere. |
VI. Recombination enhanced by replication fork blockage at the Ter site on E. coli plasmids.In order to elucidate the effects of replication arrest, which is of frequent and spontaneous occurrence, replication blocking sites have been frequently used. In E. coli, when both bi-directional replication forks are blocked at the two flanking replication blocking (Ter) sites, the next round of replication forks arrive at the Ter sites, producing a giant linear DNA molecule, whose terminus ends lead to recombination and overcome the blockage at the Ter sites through an unknown mechanism. On the other hand, eukaryotic chromosomes, and probably bacterial plasmids also, do not initiate such a second round of replication, at least under normal growth conditions. When plasmid replication is arrested at the fork blocking Ter site, it remains unknown whether recombination is enhanced or not. Therefore, we investigated whether recombination is stimulated when plasmid replication is inhibited by a 22 bp TerA site and, if the recombination is activated, what kinds of recombinational genes are required for the activation by using various rec-defective mutants as hosts. Furthermore, the recombinational genes involved in the SOS response, which is induced by plasmid replication blockage at the 22 bp Ter site, were also examined. The results were (1) the recombination is enhanced 5 – 7 fold by the fork block, (2) this enhancement is dependent on recA, recF recO, recR and recJ, but not recBC and recQ, (3) SOS induction is dependent on recF absolutely, and recJ and recQ partially, (4) the recombinational enhancement is disappeared when the 22 bp TerA sequence was replaced by the 0.6 kb original TerA -containing E. coli chromosomal fragment, which is consistent with the results of SOS induction. The last result indicates that in plasmids multiple rounds of replication do not occur, and furthermore fork blocking at the Ter site induces not only recombination but also the SOS response. This correlation suggests that there may be a common process between the initiation steps of the recombination and the SOS induction. A mechanism by which recombination and the SOS response were induced by replication blockage at the 22 bp TerA site, but not the 0.6 kb TerA containing E. coli chromosomal fragment, was proposed. |
Publication list: |
|
Serizawa, N., Horiuchi, T., and Kobayashi, T. (2004) Transcription-mediated hyper-recombination in HOT1. Genes Cells. 9, 305-315 |
|
Kobayashi, T., Horiuchi, T., Tongaonlar, T., Vu, L., and Nomura, M. (2004) SIR2 regulates recombination between different rDNA repeats, but not recombination within individual rDNA genes in Yeast. Cell 77, 441-453. |
|
Kasarjian, JK., Hidaka, M., Horiuchi, T., Iida, M., and Ryu, J. (2004) The recognition and modification sites for the bacterial type I restriction systems KpnAI, StySEAI, StySENI and StySGI. Nucleic Acids Res. 15;32(10):e82. |
|
Komori K., Hidaka M., Horiuchi T., Fujikane R., Shinagawa H., and Ishino Y. (2004) Cooperation of the N-terminal Helicase and C-terminal Endonuclease Activities of Archaeal Hef Protein in Processing Stalled Replication Forks. J Biol Chem. 279(51), 53175-53185 |
|
Watanabe, T., and Horiuchi, T. (2005) A novel gene amplification system in yeast based on double rolling-circle replication. EMBO J. 24, 190-198. |
