
 |
DIVISION OF MOLECULAR GENETICS |
| Professor: | IIDA, Shigeru |
| Research Associates: | TERADA, Rie |
| HOSHINO, Atsushi | |
| TSUGANE, Kazuo | |
| Technical Staffs: | FUKADA-TANAKA, Sachiko |
| YAMAGUCHI, Katsushi | |
| NIBB Research Fellows: | PARK, Kyeung-Il |
| CHOI, Jeong-Doo (- Mar. ’04) | |
| JSPS Postdoctoral Fellows: | EUN, Chang-Ho |
| COTSAFTIS, Olivier (Oct. ’04 -) | |
| JIANG, Gonghao (Nov. ’04 -) | |
| Postdoctoral Fellow: | JOHZUKA-HISATOMI, Yasuyo |
| Visiting Scientist: | KOUMURA, Toshiro |
| Graduate Students: | OHNISHI, Makoto 1) |
| SHIMATANI, Zenpei 1) | |
| ONODA, Shiho 1) | |
| TAKAGI, Kyoko 2) | |
| Technical Assistants: | MORITA, Yasumasa |
| NAGAHARA, Miki (- Mar. ’04) | |
| SAITO, Miho (- Aug. ’04) | |
| ASAO, Hisayo | |
| IKEGAYA, Kyoko | |
| MATSUMOTO, Miwako | |
| SHIMAMOTO, Miki (Nov. ’04 -) | |
| HASEGAWA, Yoshinobu 3) (Nov. ’04 -) | |
| Secretary: | SANJO, Kazuko |
| 1) Graduate University for Advanced Studies | |
| 2) Graduate School of Hokkaido University | |
| 3) OKAZAKI Silver Employment Agency | |
|
The main interest of the group is in understanding the biology of the dynamic genome, namely, genome organization and reorganization and its impact on gene expression and regulation. We are also characterizing various aspects of genetic and epigenetic gene regulations particularly on flower pigmentation of morning glories. In addition, we are undertaking reverse genetic approaches in order to elucidate the nature of dynamic genome in rice, a model plant for cereals. |
I. Spontaneous mutants in morning glories. |
|
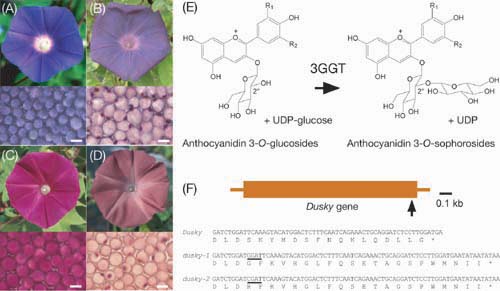
Considerable attention has recently been paid to the morning glory genus Ipomoea because of the experimental versatility of its floral biology including the genetics of floral variation, flavonoid biosynthesis, and transposon-induced mutations. The genus Ipomoea includes about 600 species distributed on a worldwide scale that exhibit various flower morphologies and pigmentation patterns. Among them, three morning glories, Ipomoea nil (the Japanese morning glory), Ipomoea purpurea (the common morning glory), and Ipomoea tricolor, were domesticated well as floricultural plants, and many mutants displaying various flower pigmentation patterns were isolated. The wild-type I. nil displays blue flowers (Figure 1A) that contain the peonidin (3’ methoxyl cyanidin) derivative named Heavenly Blue Anthocyanin or HBA. I. nil had been introduced into Japan from China approximately in the 8th century as a medicinal herb, the seeds of which were used as a laxative, and the plant became a traditional floricultural plant in Japan around the 17th century. The plant has an extensive history of genetic studies, and a number of its spontaneous mutants related to the color and shape of the flowers have been isolated. Genetic studies on the color of I. nil have shown that blue flower coloration was mainly controlled by two genetic loci, Magenta and Purple Recessive magenta and purple mutants bloom magenta and purple flowers, respectively, and double mutants carrying both magenta and purple alleles display red flowers (Figure 1C). The Magenta gene encodes flavonoid 3’-hydorxylase, which hydroxylates the 3’ position of the B-ring of anthocyanidin precursors. The Purple gene encodes a vacuolar Na+/H+ antiporter called
InNHX1 that increases the vacuolar pH during flower opening, causing a shift towards the bluer coloration. Among the various colors of I. nil flowers, the most favorite hue for Japanese floriculturists has been reddish-brown or purplish-grey petals (Figure 1B and D) since the early 19th century, and the flower coloration is mainly caused by recessive dusky mutations. We noticed that the petals in all dusky In the purple mutant deficient in the InNHX1 gene for the vacuolar Na+/H+ antiporter, the vacuolar alkalization occurs only partially, and reddish-purple buds become purple open-flowers. While most of the plant NHX genes characterized are generally expressed in leaves, stems and roots and induced by NaCl treatment, the InNHX1 gene is predominantly expressed in the flower limbs at around 12 hour before flower-opening. It is expressed very scarcely in leaves, stems and roots, and no induction occurs in response to NaCl treatment. We identified a novel vacuolar Na+/H+ antiporter gene InNHX2, which is expressed in leaves, stems and roots and is induced in response to NaCl treatment. In addition, relatively higher expression of InNHX2 was observed in the flower limbs shortly before flower-opening. We also discovered that both the InNHX1 and InNHX2 proteins could catalyze both Na+ and K+ transport into vacuoles. These results suggest that InNHX2 performs dual functions: to confer salt tolerance on the plant and to promote partial vacuolar alkalization in the petals. |
 |
|
Fig.1. Flower phenotypes and the Dusky gene for the 3GGT enzyme in I. nil. A-D. Flower phenotypes (above) and microscopic photographs of adaxial epidermal cells of flower petals (below). (A) The wild-type line with the Magenta, Purple, and Dusky alleles. (B) The line with the Magenta, Purple, and dusky-1 alleles. (C) The line with the magenta, purple, and Dusky alleles. (D) The line with the magenta, purple, and dusky-1 alleles. Scale bars in microscopic photographs indicate 30 μm. E. Reaction mediated by the Dusky gene products, 3GGT. F. The 3GGT gene and the dusky mutations. The large vertical arrow indicates the site of 4-bp insertions (above), and the 4-bp insertions underlined in the dusky mutants (below) result in frameshift mutations. |
II. Modification of endogenous natural genes by homologous recombination in rice. |
|
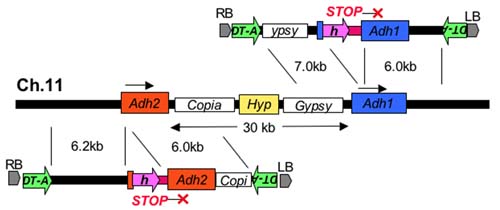
Rice (Oryza sativa L.) is an important staple food for more than half of the world’s population and a model plant for other cereal species. We have developed a large-scale Agrobacterium-mediated transformation procedure with a strong positive-negative selection and succeeded in efficient and reproducible targeting of the Waxy gene by homologous recombination without concomitant occurrence of ectopic events, which must be an important first step for developing a precise modification system of the genomic sequences in rice. By improving our transformation procedure further, we are attempting to modify the Adh1 and Adh2 genes, which belong to a small multigene family and reside adjacent to repetitive Copia- and Gypsy-like retroelements (Figure 2). The Adh1 and Adh2 genes reside on chromosome 11 in the same orientation with the 30 kb of interval, and the coding sequences of Adh1 and Adh2 are similar to each other. The results obtained indicate that the gene targeting by homologous recombination occurred more efficiently in Adh2 than that in Adh1. |
 |
|
Fig.2. Strategy for gene targeting of the Adh1 and Adh2 genes in rice. The symbols h and DT-A on the T-DNA regions of the vectors used indicate the positive and negative selection markers, respectively. The hypothetical gene flanked by the retroelements on chromosome 11 is indicated by Hyp. |
III. Characterization of a mutable virescent allele in rice. |
 |
|
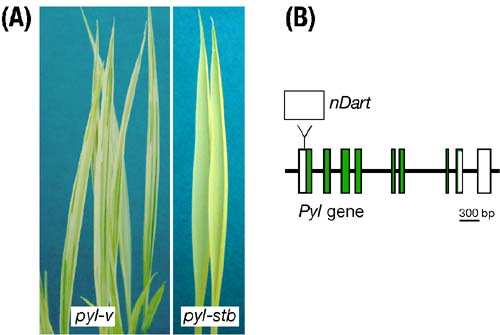
Fig.3. A. Leaf phenotypes of the mutable pyl-v and the stable pyl-stb alleles. B. Structure of the pyl-v allele. The white and green boxes represent the Pyl exons and coding region, respectively. The upper box indicates nDart1. |
|
Leaves of seedlings in the virescent mutant of rice are initially pale-yellow green due to partial deficient in chlorophyll and gradually become green with the growth of the mutant. We have been characterizing a spontaneous mutable virescent allele, pale-yellow leaf-variegated (pyl-v), conferring pale yellow leaves with dark green sectors in its seedlings (Figure 3A). The pyl-v mutant was isolated among progeny of a hybrid between indica and japonica rice plants. The leaf variegation is regarded as a recurrent somatic mutation from the recessive pale-yellow allele to the dark green revertant allele. From the pyl-v line, we also obtained a stable pyl-stb (pyl-stable) line that exhibits pale-yellow leaves without variegation (Figure 3A), which appeared to carry no active autonomous element acting on the nonautonomous DNA element inserted into the Pyl gene.The availability of the genomic sequences of both japonica and indica subspecies facilitates map-based cloning of the pyl-v allele. We identified an active nonautonomous DNA transposon of about 0.6 kb, named nDart1 (nonautonomous DNA-based active rice transposon one), in the untranslated exon 1 of the Pyl gene on chromosome 3 (Figure 3B), and excision of the new DNA transposon from the pyl gene appears to be responsible for conferring the leaf variegation. We also showed that the transposition of nDart1 could be controlled under natural growth conditions. No somaclonal variation is likely to occur in mutant lines induced by our newly characterizing endogenous element, because no tissue culture has been involved in its activation. In this respect, it is important to emphasize here that tissue culture is necessary in all of the currently available rice reverse genetic approaches including transposon tagging systems employing exogenous or endogenous transposons. We are currently attempting to develop a novel transposon tagging system in rice. |
Publication List: |
|
Yoshida, H., Akimoto, H., Yamaguchi, M., Shibata, M., Habu, Y., Iida, S. and Ozeki, Y. (2004) Alteration of methylation profiles in distinct cell lineages of the layers during vegetative propagation in carnation (Dianthus caryophyllus). Euphytica 135, 247-253. Terada, R., Asao, H. and Iida, S. (2004) A Large-scale Agrobacterium-mediated transformation procedure with a strong positive-negative selection for gene targeting in rice (Oryza sativa L.). Plant Cell Reports 22, 653-659. Iida, S., Morita, Y., Choi, J.D., Park, K.I. and Hoshino, A. (2004) Genetics and epigenetics in flower pigmentation associated with transposable elements in morning glories. Adv. Biophys. 38, 141-159. Toki, K., Saito, N., Morita, Y., Hoshino, A., Iida, S., Shigihara, A. and Honda, T. (2004) An acylated pelargonidin 3-sophoroside from the pale-brownish red flowers of Ipomoea nil. Heterocycles< 63, 1449-1454. Iida, S. and Terada, R. (2004) A tale of two integrations, transgene and T-DNA: gene targeting by homologous recombination in rice. Curr. Opin. Biotechnol. 15, 132-138. Park, K.I., Choi, J.D., Hoshino, A., Morita, Y. and Iida, S. (2004) An intragenic tandem duplication in a transcriptional regulatory gene for anthocyanin biosynthesis confers pale-colored flowers and seeds with fine spots in Ipomoea tricolor. Plant J. 38, 840-849. Ohnishi, M., Fukada-Tanaka, S., Hoshino, A., Takada, J., Inagaki, Y. and Iida, S. (2005) Characterization of a novel Na+/H+ antiporter gene InNHX2 and comparison of InNHX2 with InNHX1, which is responsible for blue flower coloration by increasing the vacuolar pH in the Japanese morning glory. Plant Cell Physiol. (in press). Morita, Y., Hoshino, A., Kikuchi, Y., Okuhara, H., Ono, E., Tanaka, Y., Fukui, Y., Saito, N., Nitasaka, E., Noguchi, H. and Iida, S. (2005) Japanese morning glory dusky mutants displaying reddish-brown or purplish-grey flowers are deficient in a novel glycosylation enzyme for anthocyanin biosynthesis, UDP-glucose:anthocyanidin 3-O-glucoside-2&rdquo-O-glucosyltransferase, due to 4-bp insertions in the gene. Plant J. (in press). |
