
 |
LABORATORY OF NEUROPHYSIOLOGY |
| Associate Professor: | WATANABE, Eiji |
| JST Technical Staff: | TAKEUCHI, Kazumi |
|
When the correct balance between water and sodium level in the body fluid has been broken, terrestrial animals feel water and salt appetite or satiety, and these perceptions subsequently induce the animal behaviors referred to as ingestion or aversion. Our research is focusing to understand molecular and neural mechanisms underlying the animal behaviors essential to homeostasis of the body fluid. To explain the properly regulated animal behaviors, neurobiologists have postulated the existence of both osmoreceptors and specific sodium receptors in the brain. However, the molecular entities of osmoreceptors and specific sodium receptors have long been enigma. In 2000, we first clarified by using gene-targeting technology that Nax sodium channel is a probable candidate for the specific sodium receptor in the brain. Nax sodium channel had long been classified as a subfamily of voltage-gated sodium channels (NaChs) that serve to generate action potentials in electrically excitable cells such as neuronal and muscle cells. Comparing with the other NaChs, however, Nax channel has unique amino acid sequences in the regions, which are known to be involved in ion selectivity and voltage-dependent activation and inactivation, suggesting that it must have specific functional properties. To clarify the functional role of Nax channel, Nax-gene deficient mice were generated by gene-targeting technique and the physiological phenotypes have been examined. Behavioral studies suggested that the Nax channel plays an important role in the central sensing of body-fluid sodium level and regulation of salt intake behavior. Nax-deficient mice ingest hypertonic sodium chloride solution in excess in comparison with wild type-mice. LacZ reporter gene knocked into Nax-gene locus revealed that Nax gene is expressed in the circumventricular organs, which is the specialized central organs involved in sensing of sodium concentration and osmosity in the body fluids. |
 |
|
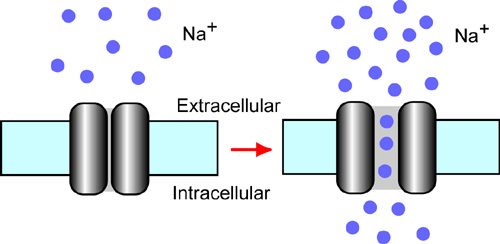
Figure 1. Nax is a sodium channel sensitive to extracellular sodium level. When the extracellular sodium concentration is increased, Nax channel opens the gate pore and generates the sodium ion influx into the cells. This view was hypothesized by ion-imaging studies. |
|
In 2002, sodium ion imaging and electrophysiological studies using cultured cells derived from the subfornical organs demonstrated that Nax channel is an extracellular sodium-level sensitive sodium channel (Fig. 1). Further, we found that Nax channel is expressed in non-myelinating Schwann cells and alveolar type II cells in addition to the cells in the circumventricular organs. Nax channel is thus likely to be involved in reception of sodium-level in the body fluids at the circumventricular organs and sodium absorption in the visceral nervous system and in the lung. In 2003, we found in collaboration with Prof. Yamamoto’s group of Osaka University that the peripheral nervous system has only subtle effects on the higher preference for sodium chloride as observed in the mutant mice. The results suggest that the mutant phenotype is mainly due to the lack of Nax channel in the central nervous system. |
 |
|
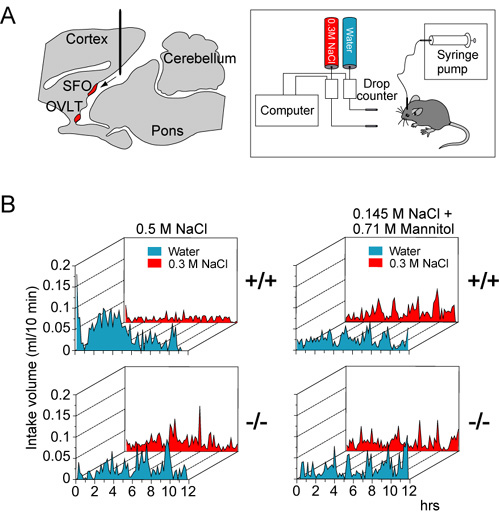
Figure 2 Nax-knockout mice are insensitive to increases of the sodium level in the cerebrospinal fluid. (A) Left, Location of the cannula for intracerebroventricular infusions. The tip of the cannula was positioned at the lateral ventricle. Right, a schematic representation of the experimental set up for the two-bottle test. Two drinking tubes were presented to free-moving mice infused with sodium solutions into the ventricle for 12 hrs. (B) Averaged time course of water and saline (0.3 M NaCl) intake in wild-type (+/+) and knockout (-/-) mice during intracerebroventricular infusions of a hypertonic (0.5 M) NaCl solution, or hypertonic mannitol (0.145 M NaCl + 0.71 M mannitol) solution. Each point shows the average quantity per 10-min period. |
|
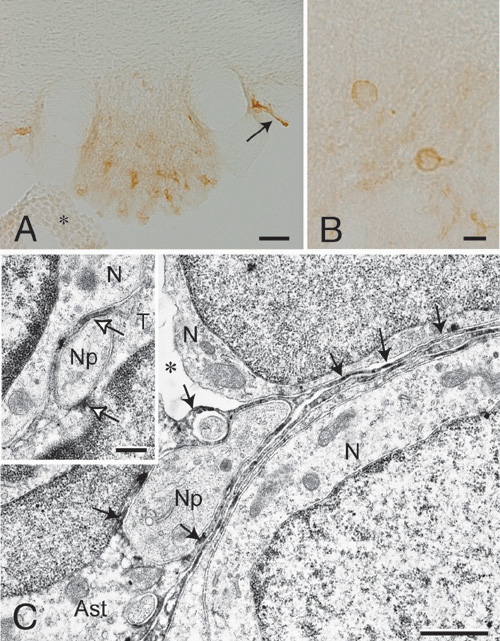
In this year of 2004, we developed an automatic measurement equipment for intake volume of drinking solutions (Fig. 2A, right). Using this equipment, we showed that the subfornical organ is the principal site for the control of salt-intake behavior, where the Nax channel is the sodium-level sensor. Infusion of a hypertonic sodium solution into the cerebral ventricle induced extensive water intake and aversion to saline in wild-type animals but not in the knockout mice (Fig 2B, left). Importantly, the aversion to salt was not induced by the infusion of a hyperosmotic mannitol solution with physiological sodium concentration in either genotype of mice (Fig. 2B, right).When Nax cDNA was introduced into the brain of the knockout mice with an adenoviral expression vector, only animals which received a transduction of the Nax gene into the subfornical organ among the circumventricular organs recovered salt-avoiding behavior under dehydrated conditions. These results clearly show that the subfornical organ is the center of the control of salt-intake behavior in the brain, where the sodium-level-sensitive Nax channel is involved in sensing the physiological increase in the sodium level of body fluids. Currently, in order to understand how the subfornical organ translates extracellular sodium-level sensed by Nax channel to the neural activities, we identified subcellular localization of Nax channel in the subfornical organ. Immuno-electronmicroscopic and double immunostaining studies clearly showed that Nax channel was exclusively localized to perineuronal lamellar processes extended from astrocytes and tanycytes in the organs (Fig. 3). Importantly, glial cells derived from the subfornical organ were capable of sensing extracellular sodium-level, as analysed by ion-imaging method. In addition, we found that the Nax-expressing glial cells enveloped GABAergic interneurons in the subfornical organ. Finally, in the subfornical organ, neuronal population activated by water deprivation was different from GABAergic interneurons, as monitored by Fos immunoreactivity. Together with previous observation that the subfornical organ of Nax knockout mice is hyperactive under water deprivation, these results indicate that the glial Nax channel senses increased sodium-level in the body fluid and inhibit the subfornical organ neuronal activity through control of GABAergic inhibitory interneurons. A novel communication style utilized the sodium-level sensitive sodium channel appears to occur between inexcitable glial cells and excitable neural cell. Since we first reported aberrant behaviors found in Nax knockout mice, a series of our studies have clarified that Nax channel is a sodium-level sensitive sodium channel playing an essential role in the sodium-sensing of the circumventricular organs and in the control of salt-intake regulation. These works identified the molecular entity of the brain sodium sensor, which has long been hypothesized as one of the important physiological issues. In this year, we newly demonstrated that the primary subcellular locus sensing sodium-level is perineuronal glial processes. This finding suggest that neuron-glia complex plays a key role on the sodium sensing in the circumventricular organs. We are now trying to construct functional expression systems of Nax sodium channel using various heterologous cell lines. The heterologous expression system will provide us useful information on the channel characters of Nax channel. |
 |
|
Figure 3. Nax channel is localized to the perineuronal processes of astrocytes and tanycytes in the subfornical organ. (A and B) Coronal tissue sections of the subfornical organ are stained with anti-Nax antibody. Immunopositive signals are widely observed within the subfornical organ (A) and most of intensive signals are accumulated in the shape of ring (B). An arrow indicates immunopositive tanycyte-layer peeled off from the subfornical organ and an asterisk indicates the choroid plexus. (C) Immuno-electron microscopic studies using anti-Nax antibody. A core region of the subfornical organ is shown. Neurons and their processes are surrounded by immunopositive thin processes of astrocytes. In inset of C, ventricular surface of the subfornical organ is shown. A neuronal process is enveloped with immunopositive thin processes of a tanycyte. Arrows point at immunopositive signals. V, ventricle; N, neuron; S, synapse; T, tanycyte; Ast, astrocyte; Np, neural process; An asterisk in C is an artificial void region occurred during fixation or staining procedures. Scale bars, 50 μm for A, 10 μm for B, 1 μm for C and 0.4 μm for inset. |
Publication List |
|
Hiyama, T.Y., Watanabe, E., Okado, H., and Noda, M. (2004) The subfornical organ is the primary locus of sodium-level sensing by Nax sodium channels for the control of salt-intake behavior. J. Neurosci. 24, 9276-9281 Niisato, K., Fujikawa, A., Komai, S., Shintani, T., Watanabe, E., Sakaguchi, G., Katsuura, G., Manabe, T. and Noda, M. (2004) Age-dependent enhancement of hippocampal LTP and impairment of spatial learning through the ROCK pathway in protein tyrosine phosphatase receptor type Z-deficient mice. J. Neurosci. in press |
