
 |
DIVISION OF BEHAVIORAL BIOLOGY (ADJUNCT) |
| Professor (Adjunct): | MORI, Yuji |
| Associate Professor (Adjunct): | TSUKAMURA, Hiroko |
| Research Associate: | IMAMURA, Takuya |
| NIBB Research Fellow: | IKEMURA, Ryota |
| Graduate Student: | TAKEUCHI, Motoki 1) |
| 1) Nagoya University | |
|
In mammals, several social behaviors are dependent on sex. Cooperative regulation of many autosomal genes as well as those on the sex chromosomes constitutes their differences. We are currently investigating the epigenetic status of the critical brain subareas responsible for the sex difference of the behaviors by analyzing genomic DNA methylation patterns. |
DNA methylation of nuclear receptors |
|
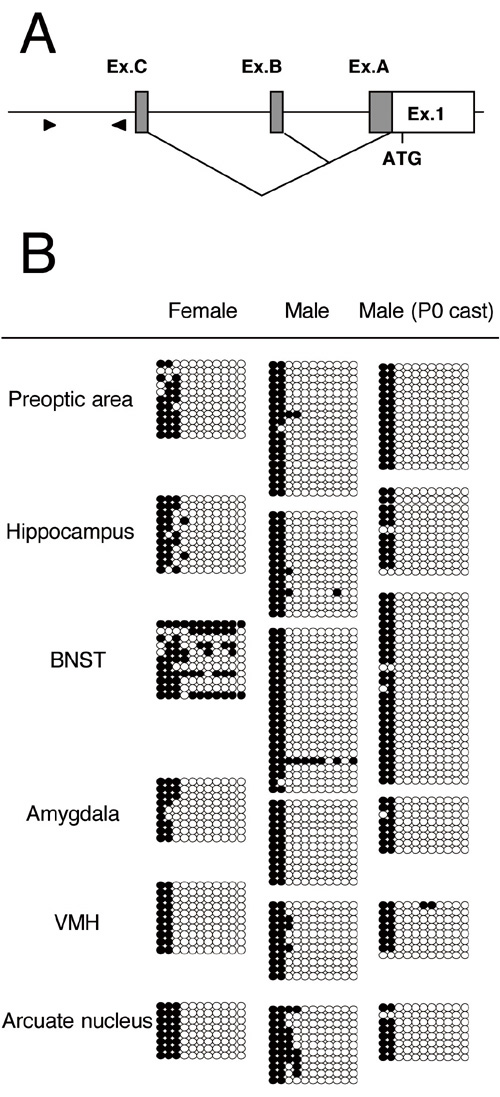
In mammals, DNA methylation, mainly occurring on CG dinucleotides, is a fundamental mechanism that differentiates the gene expression pattern in respective cell. DNA methylation is a restraint of the pluripotency because once the patterns are established during development it is maintained through cell division. On the other hand, for example, some fishes that contain much less methylation activity are found to easily and reversibly change the sex status according to the environmental context. In rodents, endocrine disturbance at the fetal and/or postnatal stages irreversibly changes the behaviors such as the lordosis (in females) and the mounting (in males) after the pubertal stages that are normally dependent on genetic sex. In some cases, lordosis can be observed even in males and vice versa. These clearly indicate that sex-dependent patterns of behaviors are not directly dependent on “sex-specific” genes but rather established through epigenetic processes. The sex-dependent patterns of behaviors must be acquired through highly irreversible processes during development. We hypothesize that the long-term effects of the sex steroid at the perinatal stages on behaviors after puberty are somehow marked at the genome level. In this year of 2004, we report herein the DNA methylation pattern of steroid receptor genes (estrogen receptor α, ERα; androgen receptor, AR; progesterone receptor, PR) in the male and female brain subareas. In general, the binding affinity of the transcription factors is reduced by hypermethylation that are frequently observed on promoter region of either ERα, AR or PR in tumor cells (Fig. 1). We analyzed the DNA methylation status of their 5’-flanking regions in the rat using the bisulfite sequencing method. In either of the genes, hypomethylated status was observed between –200 and +1 (in relation to the transcription start site) in preoptic area, bed nucleus of the stria terminalis (BNST), ventromedial hypothalamic nucleus (VMH), hippocampus, arcuate nucleus, and amygdala, suggesting that the core region of the promoters are maintained hypomethylated in normal tissues in contrast to the tumor cells. We also found that there exist the regions between –1,000 and –200, in which DNA methylation patterns differed depending on brain subarea. For ERα, no differences are found within male brain subareas examined. In contrast, hypermethylation was specifically observed in the BNST of female rats (Fig. 2). Similarly, we discovered the sex-dependent pattern of DNA methylation in the AR and PR, where hyper- and hypomethylation was found in VMH of female and male, respectively. |
 |
|
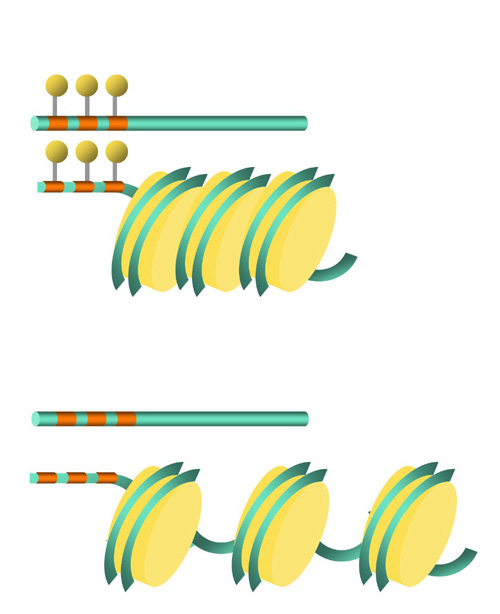
Fig. 1. Schematic representation of DNA methylation and chromatin structure. Upper panel shows the inactive state that reduces the transcriptional activity of the associated gene. Lower panel shows the DNA methylation-free state that allows DNA binding proteins, including transcription factors, accessible to the cis-regulatory element. Lollipops denote the methylated cytosines. |
|
Several reports described the significance of gonad-derived factors, such as testosterone, at the perinatal stage on brain masculinization. To determine if the sex-dependent DNA methylation status of nuclear receptors is established by gonadal factors, we made use of adult male rats castrated at the onset of birth. DNA methylation pattern of AR in VMH was reversed to that observed in the females, indicating that the epigenetic status follows the genetic sex through endocrine pathway, at least partially. However, no significant differences were found in the DNA methylation status of ERα and PR in the examined brain subregions between normal and castrated males. Therefore, these suggest that prenatal factors also function to set up the masculized/defeminized pattern of DNA methylation in the brain. In conclusion, we found the area- and sex-specific DNA methylation patterns of nuclear receptors. We are currently exploring the molecules that determine the DNA methylation pattern using the target cis-elements for differential methylation found in this study. |
 |
|
Fig. 2. DNA methylation analysis of rat estrogen receptor α A. Genomic structure of rat estrogen receptor α. Ex. denotes exon. Arrowheads indicate the relative positions of the primers for the bisulfite sequence analysis. B. Bisulfite sequencing of estrogen receptor α in adult rats. Methylation patterns of CG dinucleotides are shown. Each row of circles represents a single cloned allele with open circles for non-methylated cytosines and filled circles for methylated cytosines. P0 cast, adult male castrated at postnatal day 0. |
Publication List: |
|
Kanari, K., Kikusui, T., Takeuchi, Y., Mori, Y. (2005) Multidimensional structure of anxiety-related behavior in early-weaned rats. Behavioral Brain Res. 156, 45-52. Kikusui, T., Takeuchi, Y., Mori Y. (2004) Early weaning induces anxiety and aggression in adult mice. Physiol. Behav. 81, 37-42. Masuda, K., Hashizume, C., Momozawa, Y., Kikusui, T., Takeuchi, Y., Mori, Y. (2004) Breed differences in genotype and allele frequency of catechol O-methyltransferase gene polymorphic regions in dogs. J. Vet. Med. Sci. 66, 183-187. Kiyokawa, Y., Kikusui, T., Takeuchi, Y., Mori, Y. (2004) Modulatory role of testosterone in alarm pheromone release by male rats. Hormones Behav. 45, 122-7. Kiyokawa, Y., Kikusui, T., Takeuchi, Y., Mori, Y. (2004) Alarm pheromones with different functions are released from different regions of the body surface of male rats. Chem. Senses 29, 35-40. Kiyokawa, Y., Kikusui, T., Takeuchi, Y., Mori, Y. (2004) Partner's stress status influences social buffering effects in rats. Behavioral Neurosci. 118, 798-804. Ito, H., Nara, H., Inoue-Murayama, M., Shimada, M. K., Koshimura, A., Ueda, Y., Kitagawa, H., Takeuchi, Y., Mori, Y., Murayama, Y., Morita, M., Iwasaki, T., Ota, K., Tanabe, Y., Ito, S. (2004) Allele frequency distribution of the canine dopamine receptor D4 gene exon III and I in 23 breeds. J. Vet. Med. Sci. 66, 815-820 Masuda, K., Hashizume, C., Ogata, N., Kikusui, T., Takeuchi, Y., Mori, Y. (2004) Sequencing of canine 5-hydroxytriptamine receptor (5-HTR) 1B, 2A, 2C genes and identification of polymorphisms in the 5-HTR1B gene. J. Vet. Med. Sci. 66, 965-972. Takigami, S., Mori, Y., Tanioka, Y., Ichikawa, M. (2004) Morphological evidence for two type of mammalian vomeronasal system. Chem. Senses 29, 301-310. Estacio, M. A., Tsukamura, H., Reyes, B. A., Uenoyama, Y., I’Anson, H., Maeda, K.-I. (2004) Involvement of brainstem catecholaminergic inputs to the hypothalamic paraventricular nucleus in estrogen receptor α expression in this nucleus during different stress conditions in female rats. Endocrinology, 145, 4917-4926. Moriyama, R., Tsukamura, H., Kinosita, M., Okazaki, H., Kato, Y., Maeda, K.-I. (2004) In vitro increase in intracellular calcium concentrations induced by low or high extracellular glucose levels in ependymocytes and serotonergic neurons of the rat lower brainstem. Endocrinology 145, 2507-2515. Kinoshita, M., I’Anson, H., Tsukamura, H., Maeda, K.-I. (2004) Fourth ventricular alloxan injection suppresses pulsatile luteinizing hormone release in female rats. J. Reprod. Dev. 50, 279-285. Imamura, T., Miyauchi-Senda, N., Tanaka, S, Shiota, K. (2004) Identification of genetic and epigenetic similarities of SPHK1/Sphk1 in mammals, J. Vet. Med. Sci. 66, 1387-1393. Imamura, T., Yamamoto, S., Ohgane, J., Hattori, N., Tanaka, S., Shiota, K. (2004) Non-coding RNA directed DNA demethylation of Sphk1 CpG island, Biochem. Biophys. Res. Commun. 322, 593-600. Imamura, T., Neildez, T. M., Thenevin, C., Paldi, A. (2004) Essential role for poly (ADP-ribosyl)ation in mouse preimplantation development, BMC Mol. Biol. 5, 4. Ikemura, R., Matsuwaki, T., Yamanouchi, K., Nishihara, M. (2004) Involvement of endogenous vasopressin in high plasma osmolality-induced anorexia via V1 receptor-mediated mechanism. J. Vet. Med. Sci. 66, 951-955. |
