
 |
DIVISION OF BRAIN BIOLOGY |
| Professor: | YAMAMORI, Tetsuo |
| Research Associates: | KOMINE, Yuriko |
| WATAKABE, Akiya | |
| KITSUKAWA, Takashi | |
| Technical Staff: | OOSAWA, Sonoko |
| Technical Assistants: | MIKI, Kazuhiko |
| ISHIKAWA, Takako | |
| NIBB Research Fellow: | SI, Xuaohui |
| Postdoctoral Fellows: | SAKATA, Shuzo |
| KOMATSU, Yusuke | |
| Graduate Students: | TAKAHATA, Toru |
| SASAKI, Tetsuya | |
| TAKAJI, Masafumi | |
| NAKAMURA, Toru | |
| HIROKAWA, Junya | |
| Secretary: | HAYASHI, Hitomi |
|
Our aim of research is to understand molecular mechanisms underlying memory, formation and evolution of the brain. For one approach to understand these questions, we are studying the genes that are expressed in specific areas of the primate neocortex. We have obtained genes that showed marked differences within primate neocortical areas. Our second approach is to understand informational processing in the brain underlying learning behaviors by examining gene expression. |
I. Genes expressed in specific areas of the neocortex |
|
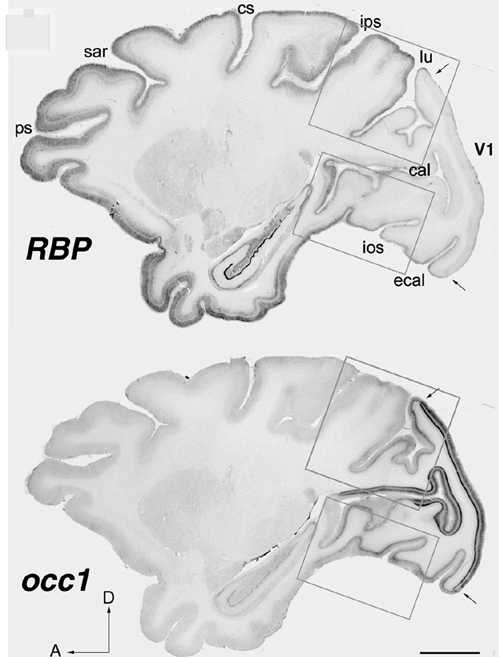
The neocortex is most remarkably evolved in theprimate and plays the major role in higher brain functions. It is divided into distinct functional and anatomical areas and has been a matter of debate what extent areas of the neocortex are genetically and environmentally deter-mined. It is also puzzling why, during the evolution of mammals, the neocortex was markedly expanded while the number of the genes in the mammal was little changed. In order to elucidate these questions, we studied gene expression within different areas of the neocortex. 1) We examined 1088 genes by macroarray analysis and found that most genes showed only less twofold difference in their expressions among the three neocortical (frontal, motor and visual) areas. Only one gene showed more than three fold difference and another one was between two and three fold difference within the three areas (Watakabe et al., Mol. Brain Res., 88, 74-82, 2001). These results suggest that the genes that are expressed among the different areas of the human neocortex are very similar. However, the question remained whether there are any genes that show marked difference within neo-cortical areas. 2) In order to answer this question, we have employed differential display methods and found three genes that indicated area specific expressions. i) One gene, designated occ1 , is specifically expressed in the occipital cortex, particularly in V1 area, in the primate brain. Furthermore, the expression of occ1 turned out to be activity dependent, because, in the monocularly deprived monkeys injected with TTX into one of the eyes, the expression of occ1 is markedly decreased in the ocular dominance columns of the primary visual cortex (V1). We also demonstrated that occ1 expression was markedly increased postnataly in V1. Interestingly, the specific expression of excitatory cells in the primary visual cortex was only observed in primates (Takahata et al., in the meeting of Soceity for Neuroscience in North America , SFN meeting, 2004). |
 |
|
Fig. 1 Expression pattern of occ1 and Rbp in the neocortex. In situ hybridization pattern of occ1 and Rbp in the primate neocortex. The two genes are expressed complimentary occ1 is markedly expressed in the visual cortex. On the other hand Rbp is specifically expressed in association areas. ps: principal sulcus, sar: superior arcute sulcus, cs: central sulcus, ips: inferior parietal sulcus, lu: lunate sulcus, cal: calcarin sulcus, ios: inferior occipital sulcus, ecal: external cal (Komatsu et al., Cerbral Cortex, 15, 96-108, 2005). |
|
ii) The other gene that showed marked difference within the neocortex, is gdf7, a member of BMP/TGF-β family, which is specifically expressed in the motor cortex of the African green monkey (Watakabe et al., J. Neurochem., 76, 1455-1464, 2001). iii) This year, we further report the third gene, Rbp (retinol-binding protein), which was preferentially expressed in association and higher areas in the neocortex (Komatsu et al., 2005). Rbp also shows characteristic features. i) Its expression is high in sensory association and higher association areas and limbic areas, but low in the primary sensory areas. Expression is complementary to that of occ1 and to parvalbumin immunoreactivity (PV-IR) in primary sensory areas. ii) In early sensory pathways, the expression is limited to superficial layers only (in particular, layer II). With progression into higher sensory areas, the expression is expanded into layers 3 and then 5. iii) In higher-order association areas, Rbp is expressed throughout all layers except layer 4. iv) This characteristic distribution of Rbp is mainly formed during postnatal development. Rbp probably regulates the concentration of retinoic acid (RA) by the delivery of retinol, which is converted into RA in cells. Although the role of RA in the mature brain is not yet known, the characteristic expression of Rbp within association areas may provide a clue to the molecular basis of the formation and function of the association areas. 3) We have also further isolated several area specific genes with RLCS (Restriction Landmark cDNA Scanning). We are now characterizing these genes to reveal the mechanisms that form neocortical areas. In summary, our studies thus far revealed the following points. (1) Genes that are specifically expressed within neocortical areas in the primate neocotex are similar overall. (2) We have identified several genes that are distinctively different among neocortical areas. (3) These genes are specific in visual, motor and association areas. (4) A gene specific in the visual cortex (occ1) is activity dependent and also postnatally regulated. (5) Rbp is expressed in association areas in a complimentary manner to the expression of occ1. (6) These results suggest that these genes may be useful markers to study the mechanisms underlying neocortical formation. |
II. Gene expression under a declarative and a non-declarative memory |
|
In order to study informational processing underlying the declarative and non-declarative memory at molecular and cellular levels in the brain, we employed c-Fos mapping techniques, for which we used gene expression of c-Fos. There have been an increasing number of studies using c-Fos as markers to examine neuronal activities ever since c-Fos induction by electrical stimulation was found. However, since many sensory stimuli per se cause c-Fos induction, we should be very careful to distinguish the c-Fos expression that is caused by learning process from that caused by sensory stimuli. For this purpose, it is necessary to use behavioral systems that are able to distinguish the difference of the two. Although a few behavioral systems in rodents have been successfully used for physiology, animal behavior and recently for analyses of knockout mice, little behavioral systems in fact distinguish the difference. Therefore, we prepared ourselves for using two behavioral systems, which represent declarative and non-declarative memory. (1) We have been collaborating with professor Yoshio Sakurai (Kyoto University) who developed an audio-visual discrimination task (AVD-task) system. In this task, a rat was asked to choose either an audio cue (a high tone or low tone) or a visual cue (a light from the right or the left) to obtain a food pellet . We found that the visual and audio tasks enhanced the specific expression of c-Fos in the visual and audio cortices, respectively. Among the early visual and auditory pathways examined, c-Fos was specifically induced in the cortices but not in the earlier pathways, suggesting the neural modulation of the neocortex depending on the types of the tasks. Interestingly, the task-dependent Fos expression was only observed in excitatory neurons in the relevant sensory cortices. |
|
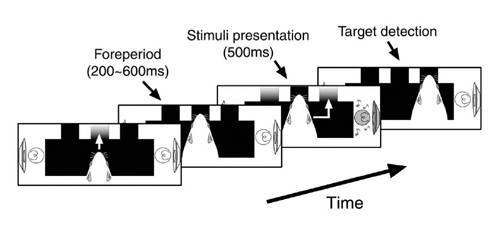
Although this AVD task system is quite powerful to analyze the problem described above and useful for studying underlying molecular and cellular mechanisms because of advantages of using rodents, one concern was that the auditory stimuli and visual stimuli are in different positions. Thus we cannot exclude the possibility that the difference between the auditory task and the visual task may not completely depend on the modality (visual Vs auditory) difference. We wanted to solve this problem by placing auditory and visual stimuli in the same position. We also use nose-poking to measure the reaction time in which a rat responds to stimuli. By using this behavioral system, we were able to confirm amodal recognition of space which means that a rat can respond to a different modality (visual or auditory) if the stimuli are in the same position and previously reported in other systems. We also confirm multisensory enhancement is indeed observed in rats. These results suggest that this new modified AVD system can be used to explore the molecular and cellular mechanisms underlying multisensory processing in rats (Sakata, 2004). |
 |
|
Fig.2 Newly developed AVD task. A rat faces to a panel with a visual cue and/or an auditory cue located in the same position, being asked to poke its nose into one of the holes to obtain reward (a pellet) (The figure is cited from Sakata et al., Exp Brain Res, 159, 409-417, 2004). |
|
Various oscillations are observed depending on brain-states. Spike wave complexes (Sws), 7-11Hz cortical oscillations with harmonics in awake but immobile rats, have been widely regarded as a model of paroxysmal activities in absence epilepsy. However, several studies have suggested that SWs in the primary somatosensory cortex are analogous to human mu rhythms. Because SWs have been frequently observed depending on vigilance levels, SWs in rats might represent normal brain-states related to the sleep-waking cycle. To elucidate behavioral contexts to induce SWs and temporal relations between SWs and neuronal ensemble activities, we recorded local field potentials (LFP) and multi-unit activities (MUAs) in the medial prefrontal cortex and electroencephalogram (EEG) in the bilateral regions of rats. Long-term recordings of EEG revealed that SWs were prominently generated in frontal and parietal regions and that SWs frequently followed non-REM sleeps. Occurrence probabilities of SWs significantly increased after the rats performed cognitive tasks. Our results suggest that SWs are one of the brain-state-specific oscillations rather than pathological activities. We also observed that MUAs were organized into phase-locked patterns in cycles of these oscillations. MUAs recorded from electrodes apart to each other were synchronized during SWs. (Sakata et al., SFN Meeting. 2004). (2) The other task we developed is a wheel running system in which a water-deprived mouse is asked to run to obtain water in front because the wheel with the pegs is turning to the other direction (Kitsukawa et al., SFN Meeting, 2002). The pegs can be changed with various patterns as desired. The task required for the mouse thus can be regarded to represent a procedural learning. We examined a various areas of brains following to the change of the peg pattern. Among the areas examined, we found marked c-Fos expression in the striatum, cerebral cortex. The striatum, which is composed of projection neurons and several distinguished types of interneurons, is known to play an important role in a reward-based learning. The characterization of these subtypes of interneurons has been progressed. However, their roles in behavioral tasks have been little known. Combining with c-Fos mapping technique and neuronal specific markers or DiI labeling, we revealed specific activation of bilaterally projecting cortico-striatal neurons and particular types of striatal neurons upon change of steps. These results suggesting cortico-striatal circuits actively participate in complex motor learning (Kitsukawa et al., SFN Meeting, 2004). |
Publication list: |
|
Sakata S, Komatasu Y, Yamamori T. (in press) Local design principles of mammalian cortical networks. Neurosci Res. Komatsu Y, Watakabe A, Hashikawa T, Tochitani S, Yamamori T. (2005) Retinol-binding Protein gene is Highly Expressed in Higher-order Association Areas of the Primate Neocortex. Cereb Cortex. 15, 96-108 Sakata S, Yamamori T, Sakurai Y. (2004) Behavioral studies of auditory-visual spatialrecognition and integration in rats. Exp Brain Res, 159, 409-417 Ichinohe N, Watakabe A, Miyashita T, Yamamori T, Hashikawa T, Rockland KS. (2004) A voltage-gated potassium channel, Kv3.1b, is expressed by a subpopulation of large pyramidal neurons in layer 5 of the macaque monkey cortex. Neuroscience, 129, 179-185. Nakayama T, Mikoshiba K, Yamamori T, Akagawa K. (2004) Activation of syntaxin 1C, an alternative splice variant of HPC-1/syntaxin 1A, by phorbol 12-myristate 13-acetate (PMA) suppresses glucose transport into astroglioma cells via the glucose transporter-1 (GLUT-1). J Biol Chem, 279, 23728-23739. |
