
 |
LABORATORY OF CYTOSKELETON |
| Associate Professor: | OGAWA, Kazuo |
I. Dynein familyDynein family is a group of microtubule-activated axonemal and cytoplasmic ATPases that serve to convert chemical energy into mechanical energy. Axonemal dynein performs flagellar and ciliary movements. In contrast, cytoplasmic dynein transports organella precursor such as vesicular membranes in a cytoplasm. Dynein is a retrograde motor and transports the cargoes to cell center. Kinesin that is also microtubule-based motor protein is an ortho-grade motor and transports the cargoes to cell periphery. Dynein and kinesin are different proteins in terms of primary structure. However, they may be coupled partners to maintain the even distribution of motor proteins in a cell. Figure 1 shows the localization of axonemal dynein at the outer arms of sperm axonemes and cytoplasmic dynein at the mitotic apparatus of cleaving egg visualized by anti-axonemal dynein (Fragment A) antibodies. |
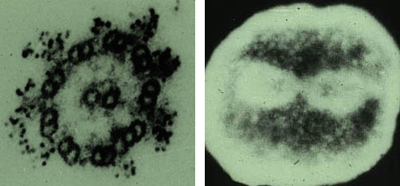 |
Figure 1. Dynein family. Axoneme (left) and Cleaving egg (right). |
II. Complex structure of dyneinDynein is a very large protein and has molecular mass up to 1 to 2 mega Da. It is complex protein consisted of heavy chains (HCs), intermediate chains (ICs), and light chains (LCs) classified by their molecular weights. Cytoplasmic dynein contains light-intermediate chains (LICs) in addition to these polypeptides. III. HCsOuter arm dynein consists of two heavy chains with ATPase activity. The motor activity is closely related to these polypeptides. Dynein is composed of three domains: stem, motor, and stalk. The first successful molecular cloning of this huge polypeptide (520 kDa) was performed in 1991. Since then cDNA clones for axonemal and cytoplasmic dyneins from a variety of organisms have been isolated and sequenced. The sequences of HCs contain, without exception, four P-loop motives referred to as ATP-binding sites in the midregion of the molecules. The NH2-terminal one-thirds corresponding to the stem and may participate in the targeting of dynein to the cargo. The probable function of the COOH-terminal one-thirds has been unknown for a while, although our previous data had shown that the middle one-thirds and the COOH-terminal one-thirds make larger domain by their intra-molecular interaction corresponding to Fragment A or motor. Yeast genome project have proposed for the new family of AAA-proteins. Taking the novel idea of AAA-protein module into consideration, there may be two AAA-modules at the COOH-terminal one-thirds and four AAA-modules at the midregion. As a consequence, dynein HCs may be a hexamer of an AAA-module as shown in Figure 2 (Vale 2000). There are two, interrupted hydrophobic heptapeptide repeats between the midregion and the COOH terminal region, forming extended flexible structure, stalk. Stalk may bind to microtubule in an ATP-dependent manner. |
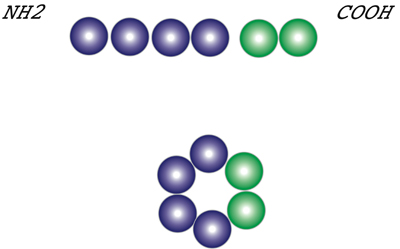 |
Figure 2. A hexamer model of an AAA-module for dynein HCs. |
IV. ICsOuter arm dynein contains three intermediate chains (IC1, IC2, and IC3) that range in molecular mass from 70 to 120 kDa. IC2 and IC3 belong to the WD-family. WD-containing ICs have been found in cytoplasmic dynein. By contrast, IC1 is a hybrid protein such that the N-terminal part is homologous to the sequence of thioredoxin and the middle part consists of three repetitive sequences homologous to the sequence of NDP kinase. The IC1-related proteins have been found in distantly related species but cytoplasmic dynein may not contain it. V. LCsSix light chains with molecular masses of 23.2, 20.8, 12.3, 11.5, 10.4, and 9.3 kDa are closely associating with outer arm dynein. We have already isolated cDNA clones of five LCs. Transmission ratio distortion is a dramatic example of non-Mendelian transmission. In mice, t-haplotype males produce dysfunctional +-sperm and normal t-sperm, leading to transmission in favor of t-sperm. Genetic studies have indicated that the t-complex responder locus, Tcr, rescues t-sperm but not +-sperm from defective products of t-complex distorter loci, Tcds. LC1 and LC3 from sea urchin sperm outer arm dynein have sequence similarities to Tctex2 and Tectex1, respectively, both of which are wild-type products of Tcds. We showed that LC1 and LC3 are able to make a 1:1 complex. Since Tcr is a member of the Smok (sperm motility kinase) family (Herrmann et al., 1999) and LC1 is phosphorylated at the activation of sperm motility in a c-AMP-dependent manner, this complex in a dynein motor molecule might be a direct target of Smok/Tcr kinase in a signal cascade that regulates sperm motility. Thus, we designated it as Smoac (sperm motility complex). Table I shows the polypeptide composition contained in outer arm dynein of sea urchin sperm flagella. Two polypeptides, α-HC and LC5 have not yet been cloned. |
|
Table I. Molecular compositions of outer arm dynein from sea urchin sperm axonemes cloned in our laboratory. |
Chains |
Accession number |
Calculated molecular weight |
α-HC |
Not cloned |
|
Β-HC |
D01021 |
511774.95 |
IC1 |
D63884 |
91622.50 |
IC2 |
D38538 |
79137.02 |
IC3 |
D28863 |
68223.32 |
LC1 |
BAA24185 |
20325.81 |
LC2 |
BAA24184 |
22201.21 |
LC3 |
AB004251 |
12535.95 |
LC4 |
BAA24152 |
12458.90 |
LC5 |
Not cloned |
|
LC6 |
AB004830 |
10325.67 |
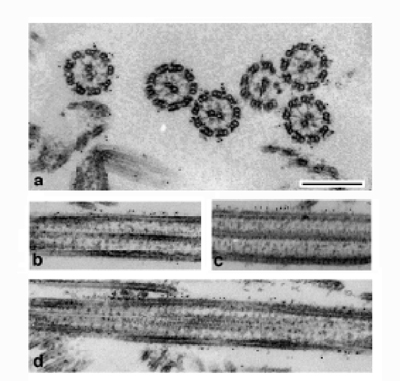
VI. Targeting of dynein to the cargoThe mechanism how dynein targets the cargoes has been gradually made clear in terms of molecules participated in. In cytoplasmic dynein, less characterized IC and LIC that are closely associated with stem of dynein are thought to target the cargoes. They are linked with dynactin complex consisted of at least ten polypeptides. It is via dynactin complex that dynein targets a receptor of cargo membranes. In flagellar and ciliary movement, outer and inner dynein arms are projected from the A-subfibers of peripheral doublet microtubules of axoneme corresponding to the cargo. They bind to the B-subfibers. They bind to the B-subfiber of neighbouring doublet microtubules in an ATP-dependent manner. Under the physiological condition, in contrast to cytoplasmic dynein, axonemal dynein is hardly detached from the A-subfibers being the cargo. In this point, axonemal dynein are different from cytoplasmic dynein in which case, the cargoes are thought to be detached from the motor after arrival to cell center to recruit the cargoes. Thus, there may be different targeting mechanism for dynein family to specific sites of the cargoes. Outer dynein arm is positioned at just 24 nm interval along axis of axonemes. The correct positioning of outer arm onto the A-subfibers is thought to be due to the outer dynein arm-docking complex (ODA-DC) that could link up end-to-end with some overlap, to form a filament with a 24 nm repeat structure (Kamiya, 2002). When Triton-model sperm were exposed to hard condition such as a high salt solution, outer arm was detached from the A-subfibers. Extracted sperm without outer dynein arm swim with a beat frequency of control sperm. Rebinding of outer dynein arm onto the A-subfibers was possible by remixing the extracted Triton-sperm with the extract under a low salt condition. Recovered Triton-model sperm swam with a normal beat frequency (Gibbons and Gibbons, 1976). Thus, a high salt extract may containing some proteins necessary for correct positioning of outer dynein arm onto the A-subfibers. During the course of characterizing proteins containing in it, we found a novel protein with molecular mass of 58 kDa designated as ap58. Immuno-electron microscopy using antibodies raised against recombinant ap58 shows that gold-particles are found at 25 nm repeat along the length of axoneme coinciding with the repeat of outer dynein arm. Thus, we conclude that ap58 is binding to in situ outer dynein arm. |
|
|
Figure 3. Localization of ap58 on outer dynein arm visualized by immuno-electron microscopy (Ogawa and Inaba) |
