
 |
DIVISION OF CELL MECHANISMS |
| Professor: | NISHIMURA, Mikio |
| Associate Professor: | HAYASHI, Makoto |
| Research Associates: | MANO, Shoji |
| Technical Staff: | KONDO, Maki |
| JSPS Postdoctoral Fellows | NITO, Kazumasa |
| KUROYANAGI, Miwa | |
| ARAI, Yuko | |
| Postdoctoral Fellows: | KAMADA, Tomoe (Apr. 1 -) |
| MUTSUDA, Michinori (Apr. 1 -) | |
| HATSUGAI, Noriyuki (Oct. 1 -) | |
| KAMIGAKI, Akane (Oct. 1 -) | |
| Graduate Students: | KAMADA, Tomoe 1) (- Mar. 31) |
| HATSUGAI, Noriyuki 1) (- Sep. 30) | |
| OGASAWARA, Kimi 2) (Apr. 1 -) | |
| Technical Assistants: | NAKAMORI, Chihiro |
| YAGI, Mina | |
| YOSHINORI, Yumi | |
| SUZUKI, Iku | |
| FUKAZAWA, Mitsue (Nov. 1 -) | |
| Secretaries: | UEDA, Chizuru |
| IYODA, Yuri (- Mar. 31) | |
| 1) Graduate University for Advanced Studies | |
| 2) Tokyo University of Marine Science and Technology | |
|
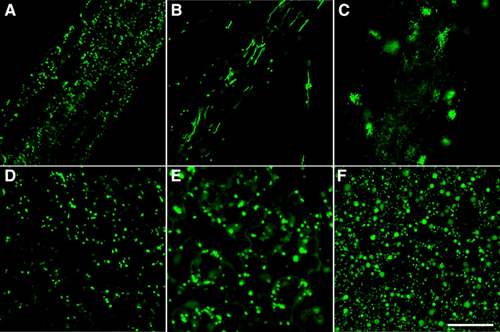
Higher plant cells contain several distinct organelles that play vital roles in cellular physiology. During proliferation and differentiation of the cells, the organelles often undergo dynamic changes. The biogenesis of new organelles may occur, existing organelles may undergo a transformation of function, while other organelles may degenerate. Because the dynamic transformation of organellar function (differentiation of organelles) is responsible for flexibility of differentiation events in higher plant cells, the elucidation of regulatory mechanisms underlying organelle transformation are currently studied in this division. I. Reversible transformation of plant peroxisomesDramatic metabolic changes which underlie the shift from heterotrophic to autotrophic growth occur in greening of seed germination. Accompanying these metabolic changes, many constitutive organelles are functionally transformed. Etioplasts differentiate into chloroplasts and mitochondria acquire the ability to oxidize glycine. Glyoxysomes, which are microbodies engaged in the degradation of reserve oil via ß-oxidation and the glyoxylate cycle, are transformed into leaf peroxisomes that function in several crucial steps of photorespiration. After the functional transition of glyoxysomes to leaf peroxisomes during the greening of pumpkin cotyledons, the reverse transition of leaf peroxisomes to glyoxysomes occurs during senescence. The functional transformation between glyoxysomes and leaf peroxisomes is controlled by gene expression, alternative splicing, protein translocation and protein degradation. We now engage in proteomic and transcriptomic analyses of the reversible peroxisomal transition in Arabidopsis cotyledons. II. Transcriptomics, proteomics and phenomics of plant peroxisomesEnzymes localized in plant peroxisomes are synthesized in the cytosol, and function after their post-translational transport into peroxisomes. Almost all of the peroxisomal matrix proteins are known to contain one of two targeting signals (PTS1 and PTS2) within the molecules. PTS1 is a unique tripeptide sequence found in carboxyl terminus of the mature proteins. The permissible combinations of amino acids for PTS1 in plant cells are [C/A/S/P]-[K/R]-[I/L/M]. In contrast, PTS2 is involved in a cleavable amino terminal presequence of peroxisomal proteins that are synthesized as a precursor protein with larger molecular mass. PTS2 consists of a consensus sequence [R]-[L/Q/I]-X5-[H]-[L]. We identified 256 gene candidates of PTS1- and PTS2-containing proteins and another 30 genes of non-PTS-containing proteins from Arabidopsis genome. Custom-made DNA microarray covering all these genes was used to investigate expression profiles of the peroxisomal genes in various organs. Statistical analyses revealed that the peroxisomal genes could be divided into five groups.One group showed ubiquitous expression in all organs examined, while the other four were classified as showing organ-specific expression in seedlings, cotyledons, roots and in both cotyledons and leaves. In parallel, we made two-dimensional protein map of glyoxysomes and leaf peroxisomes isolated from Arabidopsis Peptide MS fingerprinting analyses allowed us to identify novel proteins exists in either glyoxysomes or leaf peroxisomes. Some of these proteins contain no obvious PTS1 and PTS2. Of these, we characterized GPK1 as a novel protein kinase in glyoxysomes. Bioinfomatic analysis of Arabidopsis genome predicted the presence of 15 kinds of genes for peroxisomal biogenesis factors, called PEX genes. We comprehensively investigated whether these predicted PEX genes function in peroxisome biogenesis by generating knock-down mutants that suppress PEX gene expression by RNA-interference. Phenotypes of these mutants allowed us to identify the functional PEX genes, which can be classified into two groups, i.e. PEX genes regulating for peroxisomal morphology and peroxisomal protein import. III. Identification of novel components essential for peroxisome biogenesisTo better understand peroxisome biogenesis, we mutagenized seeds of transgenic Arabidopsis, GFP-PTS1, in which peroxisomes with normal size and number can be visualized with GFP, and isolated a number of Arabidopsis mutants having aberrant peroxisome morphology (apm mutants) based on the different pattern of GFP fluorescence (Fig. 1). |
|
|
Fig. 1 Phenotype of apm mutants. (A) to (C) root cells. (D) to (F) leaf cells. (A) and (D) show GFP-labelled peroxisomes in GFP-PTS1 as a parent plant. (B) apm1 mutants have long peroxisomes. (C) Distribution of peroxisomes is altered in apm5 mutants. (E) In apm2 mutant, the decrease of efficiency of protein transport to peroxisomes results in the observation of GFP fluorescence in the cytosol and peroxisomes. (F) apm3 mutants contain larger peroxisomes. Bar indicates 50 μm. |
|
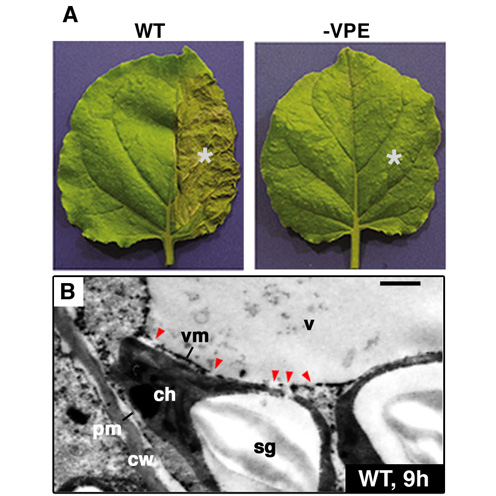
In one of these mutants, apm1, the peroxisomes are long and reduced in number, apparently as a result of inhibition of division. APM1 gene encodes DRP3A (Dynamin-related protein 3A). Interestingly, apm1 mutation also caused inhibition of mitochondrial division. Actually APM1/DRP3A protein is localized at peroxisomes and mitochondria. These findings showed that APM1/DRP3A protein is involved in both peroxisomal and mitochondrial division. With regard to another apm mutants, apm2, apm4 and apm7, the GFP fluorescence is observed in not only peroxisomes but also the cytosol, suggesting that the efficiency of protein transport to peroxisomes is decreased in these mutants. apm3 and apm6 mutants have larger peroxisomes. These phenotypes indicate that both mutants are defective in peroxisomal division. In apm5 mutants, the distribution of peroxisomes within cells changed to the one position in the cell and peroxisomes hardly move, whereas peroxisomes in GFP-PTS1 are dispersed and move frequently. Analyses of these apm mutants and identification of APM genes will identify components necessary for peroxisome biogenesis and address the regulation of its mechanism. IV. ER-derived organelles for protein storing and defense strategy.Plant cells develop various types of endoplasmic reticulum (ER)-derived structures with specific functions. ER bodies are ER-derived compartment specific to the Brassicaceae, including Arabidopsis. They are rod-shaped structures (5 μm long and 0.5 μm wide) that is surrounded by ribosomes. ER bodies can be visualized in transgenic plants of Arabidopsis expressing GFP fused with an ER retention signal (GFP-HDEL). ER bodies were widely distributed in the epidermal cells of whole seedlings. Rosette leaves had no ER bodies, but accumulated ER bodies after wounding or jasmonic acid treatment. This suggests that ER bodies function in the defense against herbivores. We isolated an Arabidopsis mutant, nai1, which has no ER bodies in whole plants. The nai1 mutant did not accumulate PYK10, a ß-glucosidase with an ER-retention signal (KDEL), in seedlings. An electron microscopic analysis showed that PYK10 was accumulated in ER bodies of the wild-type plants. This indicates that PYK10 is the main component of ER body. We cloned NAI1 gene using a positional cloning strategy. NAI1 encodes a transcription factor that has a basic-helix-loop-helix (bHLH) domain. The nai1 mutant had a single nucleotide change at an intron acceptor site of NAI1 gene. Because of this mutation, aberrant splicing of NAI1 mRNA occurs in the nai1 mutant. Transient expression of NAI1 induced ER bodies in the nai1 mutant. Two-dimensional electrophoresis and RT-PCR analyses showed that the amounts of mRNA and protein of a putative lectin in the nai1 mutant were decreased, as was the case of PYK10. These results provide direct evidence that this bHLH protein plays a role in the formation of ER bodies. V. Vacuolar processing enzyme responsible for programmed cell death in plants.Vacuolar processing enzyme (VPE) belongs to the cysteine protease family C13. This family is found in various eukaryote organisms including higher plants and animals. VPE exhibits substrate specificity toward asparagine and aspartic acid residues, the amino acid well conserved at a position in the processing sites of various vacuolar/lysosomal proteins. VPE was originally identified as an enzyme responsible for the processing and maturation of seed storage proteins in plants. We have shown that mouse VPE is responsible for the processing of lysosomal proteases, cathepsins B, H, and L, from the single-chain forms into the two-chain forms, and VPE deficient mice accumulated macromolecules in the lysosomes. Plant VPE homologues are separated to two subfamilies: one seed type and the other vegetative type. Seed-type VPE is responsible for the maturation of seed storage proteins. On the other hand, the function of vegetative-type VPE was obscure. Recently, we found a novel function of vegetative-type VPE in programmed cell death (PCD). The plant hypersensitive response (HR), a type of defense strategy, constitutes well-organized PCD. The evidences from extensive studies indicate that caspase activity is involved in plant PCD. VPE is identified as a proteinase that exhibits caspase activity. No visible lesions formed on the tobacco mosaic virus (TMV)-infected leaves of VPE-silenced tobacco plants (Fig. 2). VPE deficiency prevented the typical characteristics of PCD, such as cell shrinkage, cytoplasmic condensation, and nuclear DNA fragmentation. An ultrastructural analysis showed that the disintegration of the vacuolar membranes occurs in the leaves before visible lesions are formed. The disintegration of the vacuolar membranes continued, resulting in complete vacuolar collapse in association with plasmolysis. On the contrary, vacuoles and vacuolar membranes remained intact in the VPE-silenced plants. These results suggest that VPE is involved in vacuolar collapse, which triggers PCD. Plants evolve a death strategy mediated by a vacuolar system, which is not seen in animals. Interestingly, a vacuolar enzyme is this key player in a plant-specific cell death system. |
|
|
Fig. 2 VPE deficiency suppresses the vacuolar collapse leading to the TMV-induced hypersensitive cell death. (A) The non-silenced (WT) and VPE-silenced (-VPE) Nicotiana benthamiana plants were infected with TMV on halves of their leaves (indicated by asterisks). The photographs were taken after 24 . (B) Morphological changes of the TMV-infected regions of the non-silenced leaves (WT) at 9 hours under the electron microscope. Bar indicates 1μm. cw, cell wall; pm, plasma membrane; vm, vacuolar membrane; v, vacuole; ch, chloroplast; sg, starch granule. Red triangles indicate the disintegrated regions of vacuolar membranes. |
VI. Role of molecular chaperones on plant cell differentiation.Molecular chaperones are cellular proteins that function in the folding and assembly of certain other polypeptides into oligomeric structures, but that are not, themselves, components of the final oligomeric structure. To clarify the roles of molecular chaperones on cell differentiation, we have purified and characterized chaperonin and Hsp70s and analyzed their roles in the translocation of proteins into chloroplasts. Previously, we characterized a mitochondrial co-chaperonin (Cpn10), chloroplast co-chaperonins (Cpn20 and Cpn10) and a small heat shock protein from Arabidopsis. Recently, we started to characterize HSP90s. Their evolutional and functional characterization is now under experiments. Publication List:Hara-Nishimura, I., Matsushima, R., Shimada, T., and Nishimura, M. (2004) Diversity and functions of ER-derived compartments in plants: Are these compartments specific to plant cells? Plant Physiol. 136, 3435-3439. Hatsugai, N., Kuroyanagi, M., Yamada, K. Meshi, T., Tsuda, S., Kondo, M., Nishimura, M. and Hara-Nishimura, I. (2004) A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 305, 855-858. Hayashi, M., Yagi, M., Nito, K., Kamada, T. and Nishimura, M. (2004) Differential contribution of two peroxisomal receptors to the maintenance of peroxisomal functions in Arabidopsis. J. Biol. Chem. in press Mano, S., Nakamori, C., Kondo, M., Hayashi, M. and Nishimura, M. (2004) An Arabidopsis dynamin-related protein, DRP3A, controls both peroxisomal and mitochondrial division. Plant J. 38, 487-498. Mano, S. and Nishimura, M. (2004) Plant peroxisomes. Vitamins and Hormones in press Matsushima, R., Fukao, Y., Nishimura, M. and Hara-Nishimura, I. (2004) NAI1 gene encodes a basic-helix-loop-type transcription factor that regulates the formation of a novel ER-derived structure, the ER body. Plant Cell 15, 1536-1549. Nakaune, S., Yamada, K., Kondo, M., Kato, T., Tabata, S., Nishimura, M., Hara-Nishimura, I. (2004) A novel vacuolar processing enzyme is involved in seed coat formation at the early stage of seed development. Plant Cell in press Tamura, K., Yamada, K., Shimada, T., Hara-Nishimura, I. (2004) Endoplasmic reticulum-resident proteins are constitutively transported to vacuoles for degradation. Plant J. 39, 393-402 Usami, T., Mochizuki, N., Kondo, M., Nishimura, M. and Nagatani, A. (2004) Cryptochromes and phytochromes synergetically regulate the Arabidopsis root greening under blue light. Plant Cell Physiol. 45, 1798-1808 Watanabe, E., Shimada, T., Tamura, K., Matsushima, R., Koumoto, Y., Nishimura, M. and Hara-Nishimura, I. (2004) An ER-localized form of PV72, a seed-specific vacuolar sorting receptor, interferes the transport of an NPIR-containing proteinase in Arabidopsis leaves. Plant Cell Physiol. 45, 9-17. Yamada, K., Fuji, K., Shimada, T., Nishimura, M. and Hara-Nishimura, I. (2004) Endosomal proteases facilitate the fusion of endosomes with vacuoles at the final step of the endocytotic pathway. Plant J. in press Yamada, K., Nishimura, M. and Hara-Nishimura, I. (2004) The slow wound-response of VPE is regulated by endogenous salicylic acid in Arabidopsis. Planta 218, 599-605. Yamada, K., Shimada, T., Nishimura, M. and Hara-Nishimura, I. (2004) A VPE family supporting various vacuolar functions in plants. Physiol. Plant. in press Yoshida, K., Kawachi, M., Mori, M., Maeshima, M., Kondo, M., Nishimura, M. and Kondo, T. (2004) The involvement of tonoplast proton pumps and Na+(K+)/H+ exchangers in the change of petal color during flower-opening of morning glory, Ipomoea tricolor cv. Heavenly Blue. Plant Cell Physiol. in press |
 |