| CENTER FOR RADIOISOTOPE FACILITIES (CRF) | ||||||
|
||||||
| I. Research supporting
activity
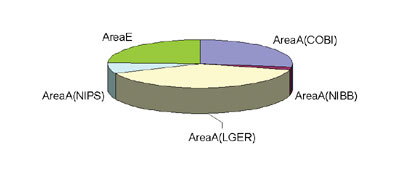
Technical and supporting staffs of the CRF maintain five controlled areas being adaptable for the law. The purchase of radioisotopes from JRA (Japan Radioisotope Association) and the transfer of radioisotope wastes to JRA is under our surveillance. In this year, we discontinued two controlled areas; NIBB (National Institute for Basic Biology)-branch and NIPS (National Institute for Physiological Science)-branch. The name of Center located in Common Building I of the area A has been changed to Common Building I (COBI)-branch. Matsuda, Iinuma, Ito, and Iida are maintaining COBI-branch and LGER (Laboratory of Gene Expression and Regulation)-branch in the area A, and Ogawa, Sawada, and Katagiri working in the area E. Users going in and out the controlled areas counted by the monitoring
system are 6,965 persons in 2003. The items in each area is shown
in Figure 1.
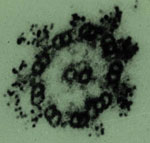
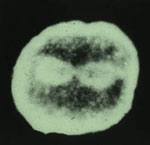
Dyneins are a group of microtubule-activated ATPases that serve to
convert chemical energy into mechanical energy and divided into axonemal
and cytoplasmic dyneins. Figure 2 shows the localization of two isoforms
of dynein in the outer arms of sperm axonemes and the mitotic apparatus
of cleaving egg visualized by anti-axonemal dynein (Fragment A) antibodies
(Fig. 2).
Dyneins are very large and range in molecular mass up to 1 to 2 mega Da. They are complex proteins containing heavy, intermediate, and light chains defined by the molecular mass. The project is the molecular cloning of polypeptides contained in outer arm dynein of sea urchin sperm flagella to understand the mechanism how dynein interacts with microtubules, resulting in producing the force. Outer arm dynein consists of two heavy chains with ATPase activity. The motor activity is closely related to this polypeptide. The first successful molecular cloning of this huge polypeptide (520 kDa) was performed in 1991. Since then cDNA clones for axonemal and cytoplasmic dyneins have been isolated in a variety of organisms. The sequences of heavy chains, without exception, contain four P-loop motives referred to as ATP-binding sites in the midregion of the molecules. Outer arm dynein contains three intermediate chains (IC1, IC2, and IC3) that range in molecular mass from 70 to 120 kDa. IC2 and IC3 belong to the WD-family. By contrast, IC1 is a hybrid protein such that the N-terminal part is homologous to the sequence of thioredoxin and the middle part consists of three repetitive sequences homologous to the sequence of NDP kinase. The IC1-related proteins are found in distantly related species. Six light chains with molecular masses of 23.2, 20.8, 12.3, 11.5,
10.4, and 9.3 kDa are associating with outer arm dynein. We have already
isolated cDNA clones of five LCs. LC1 (23.2 kDa) and LC3 (12.3 kDa)
are highly homologous to mouse Tctex2 and Tctex1, respectively, which
are encoded by the t complex region that is involved in transmission
ratio distortion (TRD), male sterility and the development of germ
cells. In this year, we found that LC1 and LC3 are able to making
sperm motility activating complex (Smoac). Our finding raises the
possibility that axonemal dynein proteins are involved in this phenomenon.
TRD may be caused by the dysfunction of multiple axonemal dynein proteins. |
||||||