| DIVISION OF PLANT DEVELOPMENTAL GENETICS | ||||
|
||||
Diversity of plant form is mostly attributable to variation of leaf and floral organs, which are modified leaves. The leaf is the fundamental unit of the shoot system, which is composed with leaf and stem. So the leaf is the key organ for a full understanding of plant morphogenesis. However, the genetic control of development of these shapes had remained unclear. Recently, studies of leaf morphogenesis has been in a turning point, after our successful application of the techniques of developmental and molecular genetics to it, using model plants, Arabidopsis thaliana (L.) Heynh. (reviewed in Tsukaya 2003). In a determinate organ, a leaf, number of leaf cells is not necessarily reflected on leaf shape or, in particular, leaf size. Genetic analyses of leaf development in Arabidopsis shows that a compensatory system(s) act in leaf morphogenesis and an increase of cell volume might be triggered by a decrease in cell number. Thus, leaf size is, at least to some extent, uncoupled from the size and number of cells by the compensatory system(s). Based on these facts, a new perspective on understanding of mechanisms for leaf morphogenesis, Neo-Cell theory, is proposed (Tsukaya, 2003). Neo-Cell theory is the Cell theory which stipulated positional cooperation of the behavior of cells. In short, Neo-Cell theory hypothesizes as follows: cells are the unit of morphogenesis; however, each cell is also controlled by factors that govern the morphogenesis of the organ of which the cells are a part. Focusing on mechanisms that govern polarized growth of leaves in a
model plant, Arabidopsis thaliana, we found that the two
genes act independently to each other on the processes of polar growth
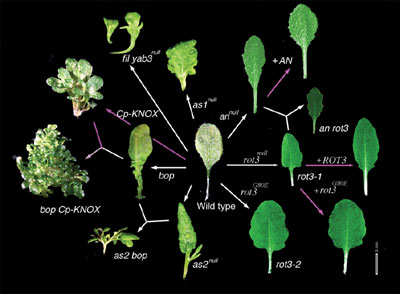
of leaves: the ANGUSTIFOLIA (AN) gene regulates width of
leaves and the ROTUNDIFOLIA3 (ROT3) gene regulates length
of leaves. The AN gene controls the width of leaf blades and the ROT3 gene controls length. Cloning of the AN gene (Kim et al.,
2002) revealed that the gene is a member of CtBP (C-terminal binding
protein) gene family which had been found from animal kingdom. In
the animal kingdom, CtBPs self-associate and act as a co-repressor
of transcription. We found that also the AN protein can self-associate
in yeast two-hybrid system (Kim et al. 2002). Furthermore, microarray
analysis suggested that the AN gene might regulate the expression
of certain genes, for example, the gene involved in formation of cell
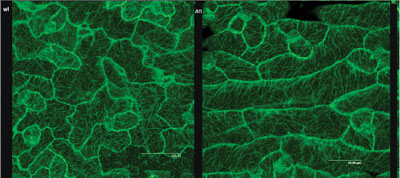
walls, MERI5. We also found that the abnormal arrangement
of cortical MTs in an-1 mutant leaf cells appeared to account
entirely for the abnormal shape of the cells. It suggested that the AN gene might regulate the polarity of cell growth by controlling
the arrangement of cortical MTs (below).
The ROT3 gene encodes a cytochrome P450. Transgenic experiments
proved that the ROT3 gene regulates leaf-length without affect
on leaf-width via biosynthesis of steroids. In relation to it, we
recently revealed that a steroid hormone, brassinosteroid, controls
both proliferation and expansion of leaf cells (Nakaya et al., 2002).
To understand the role of the ROT3/CYP90C1 enzyme in biosynthesis
of brassinosteroid, biochemical analysis of the rot3 mutants has been
carried out. As a result, we found that CYP90C1 and its closely related
homolog, CYP90D1, are involved in the critical steps of biosynthesis
of brassinolide (Kim et al., submitted). Leaf expansion is controlled not only by polar cell expansion but
also by polar cell proliferation. We have revealed that ANGUSTIFOLIA3
(AN3) gene is involved in maintenance/establishment of activity
of cell proliferation in leaf primordia. AN3 encodes a co-activator,
and is speculated to control cell cycling in leaf primordia (Horiguchi
et al., in prep.). Interestingly, the an3 shows clear “compensation”,
namely, accelerated cell expansion in relation to decrease of number
of leaf cells. Using various mutants with altered number and/or size
of leaf cells, we are currently analyzing genetic system of the compensation. Apart from the aspects of leaf expansion, we also analyzed genes for identification of leaf primordia. The AS1, AS2 and BOP genes are needed for determinate growth of leaf. We isolated and analyzed a novel mutant, bop (blade-on-petiole) mutant, which strongly enhances the as2 phenotype, in collaboration with a reaseach teams of Prof. Nam, POSTECH, Korea and Prof. Machida, Nagoya Univ. (Ha et al. 2003). Three class I knox genes, namely, KNAT1, KNAT2 and KNAT6, were misexpressed in the leaves of the bop1-1 mutant as in those of the as2 mutant. The bop1 single mutant results in ectopic, lobed blades along the adaxial side of petioles of the cotyledon and foliage leaves. The bop mutation strongly enhances the morphological abnormality of leaves when combined with the as1 or as2 mutation, resulting in repeated compound leaves. We, thus, suggest that BOP1 promotes or maintains a developmentally determinate state of leaf cells through regulation of class I knox1 genes (Ha et al. 2003). On the other hand, we are trying to identify molecular mechanisms which distinguish developmental pathway of leaves from that of shoots by studying tropical plants with 'fuzzy' morphology. For such purposes, we introduced tropical plants having queer developmental program for leaf morphogenesis, namely, Chisocheton, Guarea and Monophyllaea, as materials for the studies. The indeterminate compound leaves of members of the genus Chisocheton in Southeast Asia and of the genus Guarea in the New World and Africa, in the family Meliaceae, are unique and can develop indeterminately as a result of the activity of the leaf apical meristem, which can function very similarly to a shoot apical meristem. We performed a molecular phylogenetic study of these genera with a research team of Dr. Yokoyama, Tohoku Univ., and the result suggested that indeterminate program in the leaves of members of these two genera might have evolved only once in Meliaceae (Fukuda et al. 2003). Cloning of apical-meristem-specific genes from these species are now underway. Similarly, we are trying to clone key gene(s) which may support the indeterminate leaf growth in Monophyllaea spp. (Gesneriaceae). On the other hand, leaf shape variation is sometimes linked to variation of ploidy level. In Cayratia japonica, Vitaceae, trifoliate leaves are rarely observed in specific strains. Detailed analysis of morphological features including fertility of pollen grains and chromosome numbers in collaboration with Prof. okada, Osaka City Univ., revealed that there are two types of Cayratia japonica in Japan: fertile diploid type that sometimes develops trifoliates and sterile triploid type that develops only quiquefoliates (Okada et al., 2003). In the course of this study, we have found several curious features in diploid type of Cayratia japonica, e.g., low fertility. To understand why, analyses of closely related species are underway (Okada and Tsukaya, 2003). In addition, we are interested in environmental adaptation of leaves.
Adaptive responses of leaves against light, gravity and other environmental
factors are also analyzed in a model plant, Arabidopsis thaliana and anatomical analyses of various types of morphological adaptation
of leaves against certain kinds of environments were also performed.
To obtain clues for understanding of natural variation in plant form,
molecular systematic analyses of wild plants are also carried out
(Yokoyama et al., 2003; Tsukaya et al., 2003). So called "Evo/Devo" study of leaf morphogenesis is also one of our research project.
Publication List: Fukuda, T., Yokoyama, J. and Tsukaya, H. (2003) The evolutionary origin of indeterminate leaves in Meliaceae: phylogenetic relationships among species in the genera Chisocheton and Guarea, as inferred from sequences of chloroplast DNA. Int. J. Plant Sci. 164: 13-24. Ha, C.-H., Kim, G.-T., Kim, B.-C., Jun, J.-H., Soh, M.-S., Ueno, Y., Machida, Y., Tsukaya, H. and Nam, H.-G. (2003) The BLADE-ON-PETIOLE gene controls leaf pattern formation through regulation of meristematic activity. Development 130: 161-172. Machida, C., Iwakawa, H., Ueno, Y., Semiarti, E., Tsukaya, H., Hasebe, H., Kojima, S., and Machida, Y. (2003) Formation of a symmetric flat leaf lamina in Arabidopsis. In: Sekimura, T., Noji, S., Ueno, N. and maini, P.K. eds., Morphogenesis and Pattern Formation in Biological Systems: Experiments and Models. Springer-Verlag Tokyo, Tokyo. Okada, H., Tsukaya, H. and Okamoto, M. (2003) Intra-specific polyploidy and possiblity of occurrence of some genetic types for pollen development in Cayratia japonica, Vitaceae. Acta Phytobotanica et Geobotanica 54: 69-75. Okada, H. and Tsukaya, H. (2003) Chromosome observations of Cayratia species, Vitaceae. Acta Phytobot. Geobot. 54: 177-179. Sulpice, R., Tsukaya, H., Nonaka, H., Mustardy, L., Chen, T., and Murata, N. (2003) Enhanced formation of flowers in salt-stressed Arabidopsis after genetic engineering of the accumulation of glycine betaine. Plant J. 36: 165-176. Tsukaya, H. (2003) A simple method for collecting DNA samples in the field. Newslet. Him. Bot. 32: 15-17. Tsukaya, H. (2003) Organ shape and size: a lesson from studies of leaf morphogenesis. Curr. Opin. Plant Biol. 6: 57-62. Tsukaya, H., Fukuda, T., and Yokoyama, J. (2003) Hybridization and introgression between Callicarpa japonica and C. mollis (Verbenaceae) in central Japan, as inferred from nuclear and chloroplast DNA sequences. Mol. Ecol. 12: 3003-3011. Yokoyama, J., Fukuda, T. and Tsukaya, H. (2003) Morphological and molecular variation of Mitchella undulata Siebold et Zucc., with special reference to systematic treatment of the dwarf form from Yakushima Island. J. Plant Res. 116: 309-315. |