| DIVISION OF MOLECULAR & DEVELOPMENTAL BIOLOGY | |||||||
|
|||||||
1) Graduate School of Biostudies, Kyoto University |
|||||||
One of the research interests of this
laboratory is to understand molecular mechanism how a cell signaling
molecule, including members of Wnt, BMP and FGF families, regulates
different developmental events. A number of evidence indicated that
each signal is involved in many aspects of the vertebrate development.
For instance, we have revealed that Wnt-3a, a members of Wnt family,
plays essential roles in a number of aspects of the mouse development,
including somite development, neural crest formation and neural development.
However, cellular and molecular mechanisms how a cell signaling molecule
regulates these different events. Thus, we are focusing on precise
functional analysis of cell-to-cell signals and identification of
target genes induced by these signals. The Wnt family of genes that encode cysteine rich secreted proteins consists of at least 17 members in the vertabrate. It has already been shown that some of them are expressed and play important roles during neural development. For instance, we showed that Wnt-1 and Wnt-3a, which are expressed in the most dorsal region within the developing central nervous system, direct specification of the dorsal interneurons. Analysis of mouse embryos lacking both Wnt1 and Wnt3a and culture of explants from the neural plate indicated that these Wnt signals promote generation of the most dorsal subclass of the interneuron, called D1 and D2, at the expense of that of more ventral subclass, called D3. Wnt signaling is also implicated in the control of cell growth and differentiation during CNS development from studies of mouse and chick models, but its action at the cellular level has been poorly understand. In vitro stem cell culture is a powerful tool for examining the effect of an external signal on neural stem cells. In vitro clonal analysis has shown that, in a serum-free defined medium supplemented with FGF-2, single cells derived from the embryonic or adult brain proliferate and form floating spherical colonies, called neurospheres. Single cells derived from neurospheres self-renew to generate a new neurosphere or differentiate into neurons or glia depending on the culture condition, indicating that they have characteristics of stem cells. Thus, to understand function of Wnt signaling on the neural stem cells,
we examined the in vitro function of Wnt signaling in embryonic neural
stem cells, dissociated from neurospheres derived from E11.5 mouse
telencephalon. Conditioned media containing active Wnt-3a proteins
were added to the neural stem cells and its effect on regeneration
of neurospheres and differentiation into neuronal and glial cells
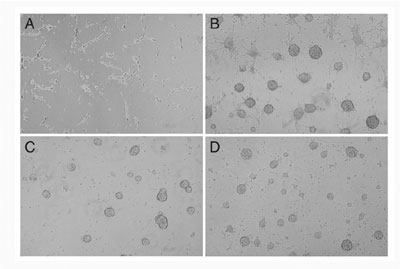
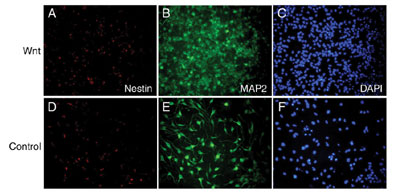
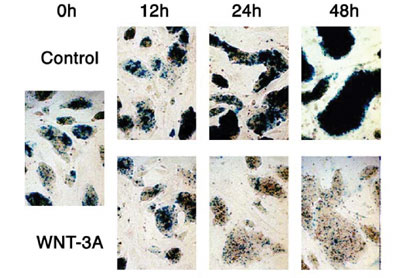
was examined. Wnt-3a proteins inhibited regeneration of neurospheres,
but promoted differentiation into MAP2-positive neuronal cells (FIG.
1). Wnt-3a proteins also increased the number of GFAP- positive astrocytes
but suppress the number of oligodendroglial lineage cells expressing
PDGFR or O4. These results indicate that Wnt-3a signaling can inhibit
the maintenance of neural stem cells, but rather promote the differentiation
of neural stem cells into several cell lineages.
Wnt-3a is also expressed in the primitive streak ectoderm during gastrulation and in the tailbud in later development of the mouse. For dissecion of the complex developmental events regulated by Wnt-3a signaling in these regions, it is important to identify genes regulated by this signal. It has already been demonstrated that T (Brachyury) is a direct target of Wnt-3a in the anterior primitive ectoderm, which is fated to give rise to the paraxial mesoderm, suggesting that Wnt-3a modurates a balance between mesodermal and neural cell fates via T. To gain more insight into roles of Wnt signaling during embryogenesis,
we searched for potential target genes of this signaling by an induction
gene trap screening in mouse ES cells. In at least three ES cell clones
among 794 clones screened, expression of beta-geo reporter genes was
dramatically changed in response to the conditioned medium of Wnt-3a
expressing cells. The expression analysis of the reporter genes in
embryos generated from these ES cell clones revealed that the spatiotemporal
expression patterns of these reporter genes were well correlated to
those of several Wnt genes. These results suggested that an induction
gene trap approach is effective for screening of target genes of Wnt
signaling during embryogenesis.
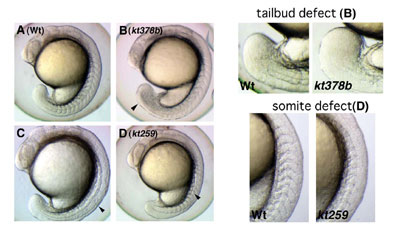
To identify genes involved in several aspects during early embryogenesis of the vertebrate, we have performed screening of zebrafish mutants induced by ENU, a chemical mutagen. Until now, we have screened 630 of F2 families and found a number of mutants whose phenotypes are different from those already reported. For instance, some of these mutants displayed defects in the somite and tailbud development. Cloning of genes that are responsible for these defects is in progress. To complement a forward genetical approach, we have also screened
genes expressed in the tailbud and presomitic mesoderm, in which somite
progenitors exist. Until now, we have identified 50 genes that are
expressed preferentially in these regions. To examine developmental
roles of these genes, functional analysis of these genes has been
performed by injecting morpholino anti-sense oligonucleotides.
Publication List: Fujino T, Asaba H, Kang MJ, Ikeda Y, Sone H, Takada S, Kim DH, Ioka RX, Ono M, Tomoyori H, Okubo M, Murase T, Kamataki A, Yamamoto J, Magoori K, Takahashi S, Miyamoto Y, Oishi H, Nose M, Okazaki M, Usui S, Imaizumi K, Yanagisawa M, Sakai J, Yamamoto TT. (2003) Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc Natl Acad Sci U S A. 100, 229-234 Muroyama Y. Kondoh H. Takada S. Wnt proteins promote neuronal differentiation in neural stem cell culture. Biochem. Biophys. Res. Commun. In press Nakagawa S, Takada S, Takada R, Takeichi M. (2003) Identification of the laminar-inducing factor: Wnt-signal from the anterior rim induces correct laminar formation of the neural retina in vitro. Dev Biol. 260, 414-425. Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. (2003) The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 8, 645-654 |