| CENTER FOR TRANSGENIC ANIMALS AND PLANTS | ||||
|
||||
| I. Research supporting activity
NIBB Center for Transgenic Animals and Plants was established in April
1998 to support researches using transgenic and gene targeting techniques
in NIBB. The center building for transgenic animals has been completed
in the end of 2003 (Figure 1). The building has a floor space of 2,300m2,
in which we can breed, develop, store and analyze transgenic, gene
targeting and mutant mice under condition of “specific pathogen
free”. It is also equipped with breeding areas for transgenic
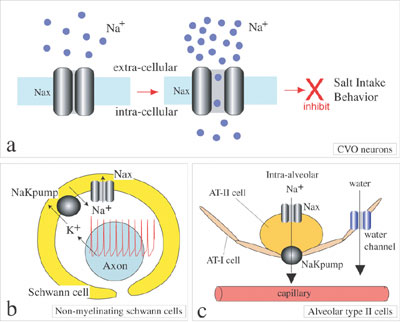
aquatic animals, birds and insects. We are studying the functional role of Nax (also called Nav2 or NaG) sodium channel in collaboration with Division of Molecular Neurobiology. Nax sodium channel was long classified as a subfamily of voltage-gated sodium channels (NaChs) that serve to generate action potentials in electrically excitable cells such as neuronal and muscle cells. Comparing with the other NaChs, however, Nax has unique amino acid sequences in the regions, which are known to be involved in ion selectivity and voltage-dependent activation and inactivation, suggesting that it must have specific functional properties. To clarify the functional role of Nax, Nax-deficient mice were generated by gene-targeting technique and the physiological phenotypes have been examined. Behavioral studies suggested that the Nax channel plays an important role in the central sensing of body-fluid sodium level and regulation of salt intake behavior. Nax-deficient mice ingest hypertonic sodium chloride solution in excess in comparison with wild type-mice. LacZ reporter gene knocked into Nax-gene locus revealed that Nax gene is expressed in the circumventricular organs (CVOs), which is the specialized central organs involved in sensing of sodium concentration and osmosity in body fluids. Sodium ion imaging and electrophysiological studies using cultured CVO neurons also suggested that Nax is an extracellular sodium-level sensitive sodium channel. Recently, we found that Nax is expressed in non-myelinating Schwann cells and alveolar type II cells in addition to the neurons and ependymal cells in the CVOs. Nax is thus likely to be involved in reception of sodium-level in body fluids at the CVOs and sodium absorption in the visceral nervous system and in the lung (Figure 2). More recently, we found in collaboration with Prof. Yamamoto’s group of Osaka University that the peripheral nervous system has only subtle effects on the higher preference for sodium chloride as ovserved in the mutant mice. The results suggest that the mutant phenotype is mainly due to the lack of Nax channels in the central nervous system. We are now trying to construct functional expression systems of Nax
sodium channel using various heterologous cell lines. The heterologous
expression system will provide us useful information on the channel
charactors of Nax.
The dopamine receptors are divided into two subgroups, referred to
as D1-like (D1, D5) and D2-like (D2, D3 and D4) receptors on the basis
of their gene structure and their pharmacological and transductional
properties. The dopaminergic system is implicated in the regulation
of the several peptide hormones in the pituitary, the modulation of
motor activity, the modulation of synaptic plasticity, and the neural
development. The dopaminergic system is also implicated in motivated
behaviors, several neurological and psychiatric disorders, such as
Parkinson’s disease and schizophrenia. Since D1-like and D2-like
receptors often work synergistically, it is necessary to delete both
D1-like and D2-like receptors in order to understand the role of dopaminergic
transmission. We have generated multiple dopamine receptor-deficient
mice by combination of single mutant mice, and are studying phenotype
of the multiple dopamine receptor mutant mice. The aim of the study is to overcome the limitations of the conventional
mouse molecular genetic approach in the functional analysis of target
genes. We substituted one critical amino acid residue of N-methyl-D-aspartate
receptor (NMDAR), leading to NMDAR activation. By our technique, we
accomplished conditional substitution of the amino acid in mice and
our mutant mice exhibited NMDAR activation and a neurological phenotype,
similar to that of mouse models for neurological disorders. The development
of our mutant mice should contribute to understanding the function
of the critical amino acid residue and the mechanism of neurological
disorders. Our new technique is vastly applicable to functional analysis
of any desired gene and should contribute to studies on the structural
and functional relationships of relevant genes. Sarcoglycans (SG) are trans-sarcolemmal glycoproteins, which associate together to form SGC and present in the sarcolemma. SGC, together with dystrophin and the dystroglycan complex comprises the dystrophin complex, which has been considered as the mechanical link between the basement membrane and the intracellular cytoskeleton for protecting the sarcolemma from mechanical stress during muscle contraction. Each of four SG subunits (a-, b-, g- and d-SG) is responsible for four respective forms of SG-deficient muscular dystrophy, sarcoglycanopathy (SGP). All of the SGs and sarcospan are absent in the sarcolemma in any form of SGP, suggesting that the SGC is not assembled, if a single subunit of the SGC is absent. To understand a physiological role of the SGC, we generated the b-SG-deficient
(BSG-/-) and g-SG-deficient (GSG-/-) mice.
The dystrophin complex isolated from the skeletal muscles of BSG-/-
mice was unstable in the absence of the SGC and sarcospan. This indicates
that SGC and sarcospan play an important role in stabilizing the dystrophin
axis connecting the basement membrane and the cytoskeleton. The BSG-/-
and GSG-/- mice developed progressive muscular dystrophy with notable
muscle hypertrophy. We found that the number of muscle fibers increased
with age and most of the fibers were regenerating fibers in their
hypertrophic muscle. Therefore, muscle hypertrophy is a consequence
of excess regenerating process but not due to fibrous and fat tissue
replacement, which has been assumed to be present in so-called pseudohypertrophy
in human diseased muscle.
| ||||
Publication List: Watanabe, U., Shimura, T., Sako, N., Kitagawa, J., Shingai, T., Watanabe, E., Noda, M., and Yamamoto, T. (2003) A comparison of voluntary salt-intake behavior in Nax-gene deficient and wild-type mice with reference to peripheral taste inputs. Brain Res. 964, 274-256 Sasaoka, T., Imamura, M., Araishi, K., Noguchi, S., Mizuno, Y., Takagoshi, N., Hama, H., Wakabayashi-Takai, E., Yoshimoto-Matsuda, Y., Nonaka, I., Kaneko, K., Yoshida, M., and Ozawa, E. (2003) Pathological analysis of muscle hypertrophy and degeneration in muscular dystrophy in gamma-sarcoglycan-deficient mice. Neuromuscular Disorders 13, 193-206 |