| DIVISION OF MORPHOGENESIS | ||||||
|
||||||
1) from the Centre for Cellular and Molecular Biology, Hyderabad, India 2) from Université de Rennes 1, Rennes, France 3) from the Institute of Basic Biological Problems, Pushchino, Russia 4) from the Biological Research Center, Szeged, Hungary 5) from Moscow State University, Moscow, Russia 6) from Plant Physiology Institute, Moscow, Russia 7) from National Center for Genetic Engineering and Biotechnology, Bangkok, Thailand 8) from Humboldt Universität Berlin, Berlin, Germany |
||||||
| The major thrust of our research efforts
is directed towards the comprehensive understanding of the molecular
mechanisms that governs the responses of plants and microorganisms
to new environmental. In particular, our attention is focused on the
perception and transduction of various stress signals, such as extreme
temperatures, osmosis and salinity. Another line of our research is
focused on the repair mechanisms from the damage to the photosynthetic
machinery under severe stress conditions. In 2003, significant progress
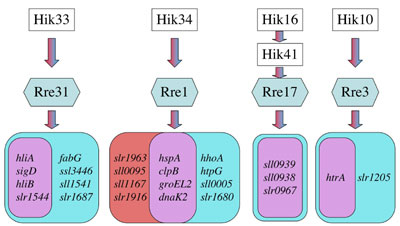
was made in the following areas. I. Discovery of four kinds of the two-component system responsible for perception and transduction of hyperosmotic and salt signals in Synechocystis. Living organisms respond and acclimate to hyperosmotic and salt stress by changing the gene expression. We previously discovered that the cyanobacterium Synechocystis sp. PCC 6803 (hereafter Synechocystis) responds differently to hyperosmotic stress and salt stress with respect to regulation of gene expression and changes in the cytoplasmic volume. These results suggested that this organism might recognize the two kinds of stress as different signals and perceived them by different sensors. To investigate the components involved in the perception and transduction of hyperosmotic and salt signals in this organism, we examined all knockout mutants for 43 histidine kinases (Hik) and 42 response regulators (Rre) by a DNA microarray technique and found, in contrast to the prediction, that the four kinds of two-component signalling pathways of Hik33/Rre31, Hik34/Rre1, Hik16/Hik41/Rre17 and Hik10/Rre3 are involved in perception and transduction of both hyperosmotic and salt signals. However, each of the two-component signaling pathways except Hik16/Hik41/Rre17 was found to regulate the expression of genes that were induced specifically by either hyperosmotic stress or salt stress, although they also regulated the expression of genes which were commonly induced by these two stress signals (Fig. 1). For example, Hik34 perceives and transduces both hyperosmotic and salt signals to the response regulator Rre1 to induce the expression of different sets of genes. Although the number of genes which are regulated by the Hik10/Rre3 pathway is small, this two-component system induces different genes in response to different stimuli. These findings clearly demonstrate that the two-component systems constitute complex signal-integration pathways rather than a simple “one stimulus-one output” scheme.
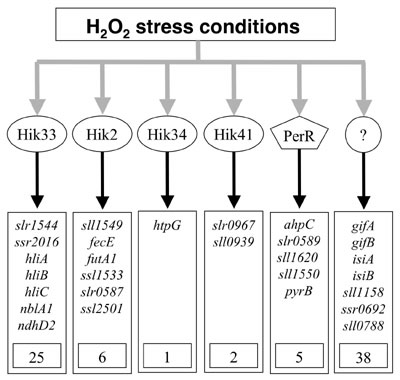
II. Discovery of four histidine kinases as sensors of H2O2 signals in Synechocystis. Hydrogen peroxide (H2O2) is produced as an inevitable consequence of aerobic life. Microorganisms detect increases in the concentration of H2O2 and regulate the expression of certain genes to enhance tolerance against this oxidative stress. DNA microarray analysis of genome-wide expression of genes revealed that incubation of Synechocystis cells with 0.25 mM H2O2 dramatically changed the pattern of gene expression. This treatment induced the expression of 77 genes and repressed the expression of 55 genes with induction factors > 4.0. A half of the H2O2-inducible genes were specifically induced by H2O2 but by no other stress, such as heat, cold, light, salt and hyperosmotic stress. These findings suggest that the oxidative stress may be distinct from the general stress. Screening for mutants in the disruptant library of histidine kinases that exhibit null response to the H2O2-inducible expression of genes identified four histidine kinases, Hik33, Hik34, Hik2 and Hik41, as sensors of H2O2 signals. They regulate the expression of 25, 1, 6, and 2 H2O2-inducible genes, respectively (Fig. 2). In Bacillus subtilis, the transcription factor PerR perceives H2O2 signals and regulates the expression of a group of H2O2-responsible genes. We also examined the genome-wide patterns of gene expression in the mutant of PerR homolog in Synechocystis by DNA microarray analysis. We found that PerR regulates the expression of five H2O2-inducible genes. These findings indicate that histidine kinases act as major sensors of H2O2 signals in Synechocystis.
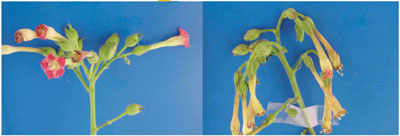
III. Multi-stress sensing by Hik33 in Synechocystis. Our previous study indicated that Hik33 is involved in the regulation of gene expression by cold and hyperosmotic stresses. We applied the DNA microarray technique to examine whether Hik33 also perceives stress signals other than cold and hyperosmotic stresses. The results clearly showed that Hik33 is involved also in the perception of salt, H2O2, and strong-light stresses. However, it does not regulate the gene expression caused by heat and nutritional stresses. Nevertheless, Hik33 regulates distinct sets of genes under different stress conditions. Therefore, it is possible that Hik33 senses the various kinds of stress to differentially regulate the gene expression in a stress-specific manner. However, it should be noted that Hik33 regulates the expression of genes which are induced commonly by various kinds of stress. Interestingly, Rre31 which is a cognate response regulator of Hik33 in the gene expression due to hyperosmotic and salt stress is not involved in the signal transduction pathway of cold and H2O2 stresses. These results may suggest that Hik33 perceives various kinds of stimulus and sorts their signals to the stimulus-specific downstream pathways. These findings suggest that the two-component signal-transduction system is more complex than a currently accepted scheme where a single sensory histidine kinase is tightly coupled with a cognate response regulator. IV. Environmental stress inhibits the repair of photosystem II by suppressing the transcriptional and translational activities. Strong light impairs the photosynthetic machinery, in particular, photosystem II (PSII), via a process known as photodamage or photoinhibition and the PSII reaction center protein D1 is most sensitive target of photodamage. However, photodamaged PSII can be repaired by replacement of the photo damaged D1 with light-dependently synthesized D1 de novo. Under natural conditions organisms are exposed to combinations of various kinds of environmental stress, such as light, salt, oxidative, heat, and cold stress, that may synergistically act on the damage to PSII. We have found that the effects in vivo of light on PSII are completely different from the effects of the other kinds of stress. Strong light induces photodamage to PSII, whereas the other kinds of stress inhibit the repair of the photodamaged PSII and do not accelerate damage to PSII directly. Results of molecular-biological analysis reveals that these kinds of stress inhibit either or both of the transcription and translation of several genes, in particular, psbA genes for D1, whose turnover is essential for the repair of PSII. These results suggest that stress inhibits the repair of PSII via suppression of the activities of both transcriptional and translational machineries. We also elucidated that the requirement of light for the repair can be explained by sustenance of the intracellular concentration of ATP via the photosynthetic transport of electrons. V. Transformation of plants to enhance the stress tolerance of reproductive organs to salt and cold stress. We showed previously that transformation with the codA gene for choline oxidase allows plants to synthesize glycine betaine (GB) and enhances their ability to tolerate various kinds of stress during germination and vegetative growth. In these years, we examined tolerance of transformed plants to salt stress at the reproductive stages, i.e., the stages at which plants are most sensitive to environmental stress. Salt-shock treatment of wild-type plants for three days resulted in the abortion of flower buds and decreased the number of seeds per silique. Microscopic examination of floral structures revealed that salt stress inhibited the development of anthers, pistils and petals. In particular, the production of pollen grains and ovules was dramatically inhibited. These effects of salt stress were significantly reduced by transformation with the codA gene, and our observations suggest that the enhanced tolerance of the transgenic plants was a result of the accumulation of GB in the reproductive organs. The cis-unsaturated molecular species of phosphatidylglycerol (PG) in chloroplasts have been implicated in the chilling tolerance of plants. We established homozygous lines of transgenic tobacco (Nicotiana tabacum) that overexpressed a cDNA for glycerol-3-phosphate acyltransferase, a key enzyme in the determination of the extent of cis-unsaturation of the PG, from a chilling-sensitive squash (Cucurbita moschata). In transgenic plants, the proportion of saturated plus trans-monounsaturated molecular species of PG increased from 24% to 65%. However, this change did not affect the architecture of the chloroplasts. Chilling stress also damaged inflorescences much more severely in transgenic plants than wild-type plants (Fig. 3). These observations allowed us to conclude that decreases in the proportion of cis-unsaturated PG enhanced the sensitivity to chilling of reproductive organs.
Publication List: (1) Original articles Szalontai, B., Kota, Z., Nonaka, H., Murata, N. (2003) Structural consequences of genetically engineered saturation of the fatty acids of phosphatidylglycerol in tobacco thylakoid membranes. An FTIR Study. Biochemistry, 42, 4292-4299. Ferjani, A., Mustardy, L., Sulpice, R., Marin, K., Suzuki, I., Hagemann, M., Murata, N. (2003) Glucosyl-glycerol, a compatible solute, sustains cell division under salt stress. Plant Physiol., 131, 1628-1637. Inaba, M., Suzuki, I., Szalontai, B., Kanesaki, Y., Los, D.A., Hayashi, H., Murata, N. (2003) Gene-engineered rigidification of membrane lipids enhances the cold inducibility of gene expression in Synechocystis. J. Biol. Chem., 278, 12191-12198. Marin, K., Suzuki, I., Yamaguchi, K., Yamamoto, H., Ribbeck, K., Kanesaki, Y., Hagemann, M., Murata, N. (2003) Identification of histidine kinases that act as sensors in the perception of salt stress in Synechocystis sp. strain PCC 6803. Proc. Natl. Acad. Sci. USA, 100, 9061-9066. Sulpice, R., Tsukaya, H., Nonaka, H., Chen, T.H.H., Murata, N. (2003) Enhanced formation of flowers and seeds in salt-stressed Arabidopsis after genetic engineering of the accumulation of glycinebetaine. Plant J., 36, 165-176. Sarcina, M., Murata, N., Tobin, M.J., Mullineaux, C.W. (2003) Lipid diffusion in the thylakoid membranes of the cyanobacterium Synechococcus sp.: Effect of fatty acid desaturation. FEBS Lett., 553, 295-298. Allakhverdiev, S.I., Mohanty, P., Murata, N. (2003) Dissection of photodamage at low temperature and repair in darkness suggests the existence of an intermediate form of photodamaged photosystem II. Biochemistry, 42, 14277-14283. Kanervo, E., Spetea, C., Nishiyama, Y., Murata, N., Andersson, B., Aro, E. (2003) Dissecting a cyanobacterial proteolytic system: efficiency in inducing degradation of the D1 protein of photosystem II in cyanobacteria and plants. Biochim. Biophys. Acta, 1607, 131-140. (2) Review articles Mikami, K., Murata, N. (2003) Membrane fluidity and the perception of environmental signals in cyanobacteria and plants. Prog. Lipid Res., 42, 527-543. Mikami, K., Suzuki, I., Murata, N. (2003) Sensors of abiotic stress in Synechocystis. Topics in Current Genetics: Plant Response to Abiotic Stress, vol. 4 (eds., Hirt, H., Shinozaki, K.), Springer, Berlin, p.103-119. |