| DEPARTMENT OF CELL BIOLOGY | ||||
|
||||
| 1) CREST, JST; 2) PREST, JST; 3) Graduate University for Advanced Studies, SOKENDAI | ||||
The pituitary-gonadal axis plays an important role
in regulating gametogenesis in vertebrates. Gonadotropins typically
act through the biosynthesis of gonadal steroid hormones which in
turn mediate various stages of gametogenesis. Their effects are particularly
profound in teleost fishes which provide excellent models for investigating
the basic hormonal mechanisms regulating gonadal sex differentiation
and gametogenesis Our research focuses on (1) the identification of
regulators and steroidal mediators involved in sex determination,
gonadal sex differentiation and gametogenesis, and (2) the mechanisms
of synthesis and action of these mediators. Although the sex-determining gene SRY/Sry has been identified in mammals, no comparable genes have been found in non-mammalian vertebrates. To identify such a sex-determining gene, a positional cloning approach is suitable. The medaka, Oryzias latipes, has two major advantages for genetic research: a large genetic diversity within the species and the existence of several inbred strains. As in mammals, sex determination in medaka is male heterogametic, although the Y chromosome is not cytogenetically distinct. Alteration of phenotypic sex with no reproductive consequences, and recombination over the entire sex chromosome pair, suggest that there are no major differences, other than a sex-determining gene, between the X and Y chromosomes. Medaka possesses a stable genetic XX/XY sex determining system. Using
positional cloning and detailed sequence analysis of BAC clones by
shotgun sequencing, we identified DMY (DM domain gene on
the Y chromosome) as a strong candidate for the sex-determining gene
of medaka. DMY encodes a protein of 267 amino acids including
the highly conserved DM domain. The DM domain was named after a related
DNA binding motif found in two proteins, dsx and mab-3,
involved in sexual development in Drosophila and C. elegans,
respectively. Two naturally occurring XY female medaka showed
different mutations in the DMY gene. One of these mutants
was found to carry a mutation causing a frameshift and premature termination
of the DMY protein. When mated, all XY offspring with the mutant Y
were female. The other mutant had a severe depression in DMY expression in the embryo and 60% of its XY offspring with the mutant
Y developed as females. More recently, we showed that a 117-kilobase
genomic DNA fragment carrying DMY was sufficient to induce
testis differentiation and subsequent male development. These loss-
and gain-of-function studies indicate that DMY is the sex-determining
gene of medaka. DMY provides the first example of a sex-determining
gene in non-mammalian vertebrates. O. curvinotus also has
the DMY gene on the Y chromosome, which is homologous to
the Y chromosome of medaka, and that DMY is expressed in
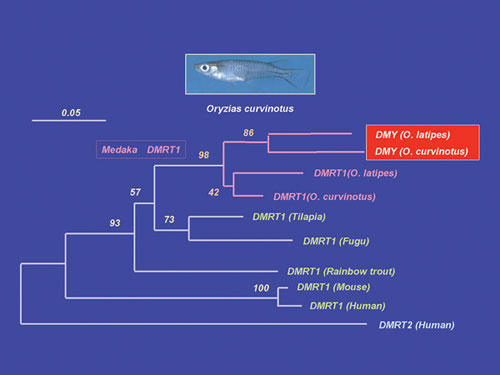
XY embryos. A phylogenetic tree based on the amino acid sequence including
the DM-domain shows that DMY was derived from DMRT1 immediately before speciation of O. latipes and O. curvinotus (Fig. 1). The branch length of DMY is longer than that of DMRT1 in the phylogenetic tree. This means that DMY has more mutations than DMRT1 and suggests that DMY accumulated these mutations after it acquired its sex-determining
function. These results suggest that a new sex-determining gene generated
by a gene duplication event in the sex chromosome tends to evolve
fast.
Nile tilapia, Oreochromis niloticus, is an excellent example of the precise nature of steroidogenic actions during gonadal sex differentiation. In this fish, all genetic female (XX) or male (XY) broods can be obtained by artificial fertilization of normal eggs (XX) and sex-reversed, pseudo male sperm (XX), or normal eggs (XX) and super male sperm (YY), respectively. Fertilized eggs hatch after 4 days at 26ÚC. On the day of hatching, primordial germ cells (PGCs), are located in the outer layer of the lateral plate mesoderm around the hind gut. At 3 days post-hatching, PGCs are located in the gonadal anlagen after the formation of the coelomic cavity in the lateral plate mesoderm rather than through active mi-gration. In tilapia, ovarian differentiation is initially marked by the appearance
of an narrow space in the stromal tissue representing the formation
of the ovarian cavity. Using all-female tilapia fries, we showed that
steroid-producing cells in XX gonads prior to and during sex differentiation
express all of the steroidogenic enzymes required for estradiol-17b biosynthesis from cholesterol. Transcripts of estrogen receptors (ER) a and b first
appear in both female and male gonads of fries at 5-10 days after
hatching. These results, together with evidence of masculinization
of genetic females by fadrozole (an aromatase inhibitor) or tamoxifen
(an estrogen receptor antagonist), strongly suggest that endogenous
estrogens act as the natural inducers of ovarian differentiation in
tilapia. In contrast, the ability of steroid-producing cells to synthesize
steroid hormones in the testes only appears at the time of testicular
differentiation, suggesting that unlike in females, in males sex steroids
do not play any major roles in testicular differentiation in tilapia.
This was further confirmed by the absence of transcripts of androgen
receptors in gonads of genetic males during sex differentiation. We
have cloned several genes which were reported to be involved in gonadal
sex differentiation of other vertebrates including Sox9, Dax1, Ad4BP/SF-1,
etc. Among these genes, DMRT1 was the only gene that was
shown to be expressed male-specifically in the gonads (Sertoli cells)
during sex differentiation, suggesting an important role for DMRT1 in testicular differentiation. Using an organ culture system for eel testes consisting of spermatogonia
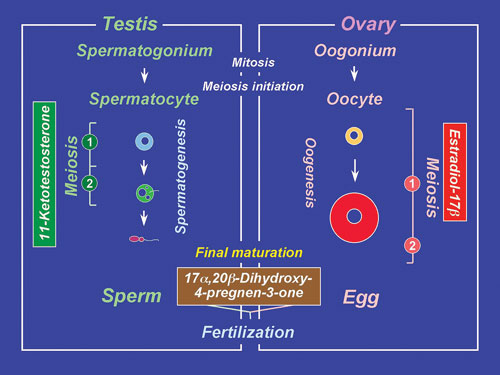
and inactive somatic cells, we have shown that the hormonal regulation
of spermatogenesis in eel testes involves the gonadotropin stimulation
of Leydig cells to produce 11-ketotestosterone (11-KT), a potent androgen
in fish (Fig. 2). In turn, 11-KT activates Sertoli cells to stimulate
the production of activin B. Addition of recombinant eel activin B
to the culture medium induced proliferation of spermatogonia, producing
late type B spermatogonia, within 15 days in the same manner as did
11-KT. cDNAs encoding two androgen receptors (ARa and ARb) have been cloned, for the first
time in any vertebrates, from eel and tilapia testes. In situ hybridization reveals that although both AR mRNAs are present in eel
testes prior to HCG injection, only ARa transcripts increase during
HCG-induced spermatogenesis suggesting that ARa and ARb play different roles in spermatogenesis.
Activin B binds to activin type I and II receptors on spermatogonia
to stimulate de novo synthesis of G1/S cyclins and CDKs leading
to the initiation of mitosis. Interestingly, cyclin A1 transcripts
are first detected in primary spermatocytes during HCG-induced spermatogenesis.
IV. Endocrine regulation of oocyte growth and maturation Gonadotropins (FSH and LH) require two major steroidal mediators, estradiol-17b and 17a, 20b-dihydroxy-4-pregnen-3-one (17a, 20b-DP) to act as critical hormones to execute oocyte growth and maturation, respectively (Fig. 2). Two cell-type models in which the thecal layer provides precursor steroids to the granulosa layer, have been demonstrated for estradiol-17b and 17a, 20b-DP production. Ad4BP/SF-1 serves as a transcriptional regulator for ovarian cytochrome P450 aromatase (P450arom) which converts testosterone to estradiol-17b in granulosa cells during vitellogenesis. LH induces a distinct shift in steroidogenesis, i.e. from estradiol-17b to 17a, 20b-DP as well as the steroidogenic enzyme genes from P450arom to 20b-hydroxysteroid dehydrogenase (20b-HSD), in the granulosa layers of ovarian follicles prior to oocyte maturation. The triggering of the steroidogenic shift by LH is manifested through two molecular mechanisms, the first being the subjugation of the expression of Ad4BP/SF-1 vis a vis P450arom, and the second, the induction of over-expression of 20b-HSD probably via CREB. Unlike estradiol-17b (genomic action), 17a, 20b-DP binds to a novel, G-protein-coupled membrane receptor (non-genomic action), leading to the de novo synthesis of cyclin B, the regulatory component of maturation-promoting factor (MPF), which activates a preexisting 35-kDa cdc2 kinase via phosphorylation of its threonine 161 by a threonine kinase (MO15), thus producing the 34 kDa active cdc2. Upon egg activation, MPF is inactivated by degradation of cyclin B. We showed that the 26S proteasome initiates cyclin B degradation through the first cut of its NH2 terminus at lysine 57. An endocrine-disrupting chemical, diethylstilbestrol (DES), a nonsteroidal
estrogen, triggers oocyte maturation in fish. The morphology (the
time course of the change in germinal vesicle breakdown) and an
intracellular molecular event (the de novo synthesis of
cyclin B) induced by DES were indistinguishable from those induced
by 17a, 20b-DP.
A synergistic action of DES on 17a, 20b-DP-induced
oocyte maturation was observed. Both 17a,
20b-DP- and DES-induced oocyte maturation
was inhibited by an antibody against the 17a,
20b-DP receptor. These results suggest
that DES may act on the 17a, 20b-DP
receptor as an agonist of 17a, 20b-DP. |
||||
Publication List: He, C.L., Du, J.L., Huang, Y.S., Lee, Y.H., Nagahama, Y. and Chang, C.F. (2003). Differential expression of androgen receptor and estrogen receptor in gonad in relation to the sex change in protandrous black porgy, Acanthopagrus schlegeli. Biol. Reprod. 69, 455-461. |