NATIONAL INSITUTE FOR BASIC BIOLOGY

National Institute for Basic Biology
DIVISION OF CELL FUSION
(Adjunct)
- Professor:
- Issei Mabuchi
- Associate Professor:
- Hiroshi Abe
- Research Associate:
- Hirotaka Fujimoto
NATIONAL INSITUTE FOR BASIC BIOLOGY

National Institute for Basic Biology
|
|
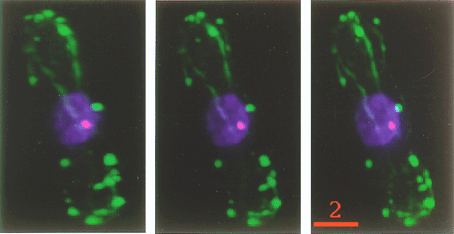
Fig. 1 3-D images of an interphase S. pombe cell. Each image is rotated by 12 degree from the neighbor. Green, F-actin. Blue, DNA. Red, spindle pole body. Bar, 2 mm. |




webmaster@nibb.ac.jp
Last Modified: 12:00, May 28, 1999