NATIONAL INSITUTE FOR BASIC BIOLOGY

National Institute for Basic Biology
DIVISION OF GENE EXPRESSION AND REGULATION II
- Professor:
- Takashi Horiuchi
- Research Associates:
- Masumi Hidaka
- Takehiko Kobayashi
- Ken-ichi Kodama
- Katsuki Johzuka
- Takehiko Kobayashi
- Post Doctoral Fellow:
- Katufumi Ohsumi 1
- Graduate Students:
- Keiko Taki
- Hiroko Urawa
- Technical Staff:
- Kohji Hayashi
- Yasushi Takeuchi
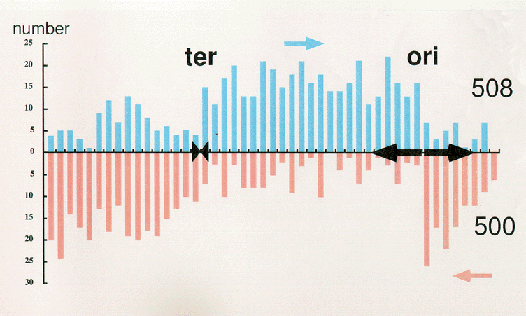
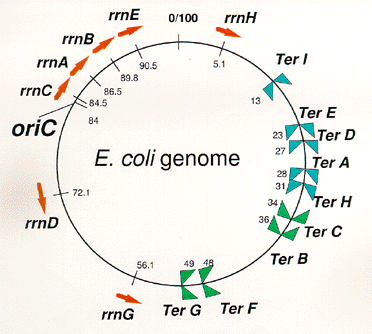
 means DNA replication terminus (Ter) site which can block replication fork approaching from the right.
means DNA replication terminus (Ter) site which can block replication fork approaching from the right.



