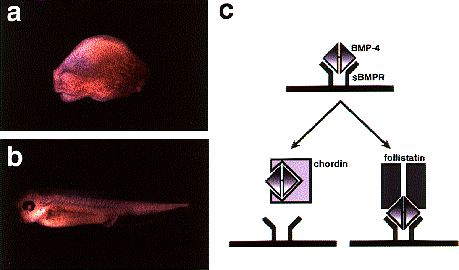
NATIONAL INSITUTE FOR BASIC BIOLOGY

National Institute for Basic Biology
DIVISION OF MORPHOGNESIS
- Professor:
- Naoto Ueno
- Associate Professor:
- Hiroshi Shibuya
- Research Associates:
- Makoto Nakamura
- Makoto Mochii
- Graduate Students:
- Hideyuki Nagaso (Hokkaido University)
- Masataka Nikaido (Hokkaido University)
- Yoshihiro Takatsu (Hokkaido University)
- Michiru Nishita (Hokkaido University)
- Katsura Sugawara (The Graduate
- University for Advanced Studies)
- Satoru Yoshida (The Graduate
- University for Advanced Studies)
- Shinichi Ohki (Hokkaido University)
- Yoshinori Tomoyasu (Hokkaido University)
- Masataka Nikaido (Hokkaido University)
- Visiting Scientists:
- Shinichi Higashijima (PRESTO, STA)
- Kiyokazu Morita (Hokkaido University)
- Fumihiko Hamada (Osaka University)
- Kiyokazu Morita (Hokkaido University)
- Technical Staffs:
- Chiyo Takagi
- Takamasa Yamamoto
- Shunichiro Iemura
- Yuko Takahashi
- Takamasa Yamamoto