NATIONAL INSITUTE FOR BASIC BIOLOGY

National Institute for Basic Biology
DIVISION OF CELL FUSION
(Adjunct)
- Professor:
- Issei Mabuchi
- Associate Professor:
- Hiroshi Abe
- Research Associate:
- Hirotaka Fujimoto
NATIONAL INSITUTE FOR BASIC BIOLOGY

National Institute for Basic Biology
|
|
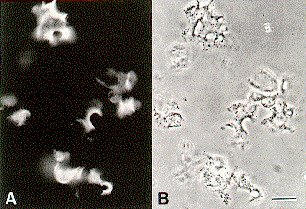
Fig. 1 Cleavage furrows of Hemicentrotus pulcherrimus eggs isolated with the isolation medium that does not contain protein phosphatase inhibitors. Fluorescence (A) and phase-contrast (B) micrographs of cleavage furrows stained with rhodamine-phalloidin are presented. Bar, 50 µm. |




webmaster@nibb.ac.jp
Last Modified: 12:00, May 28, 1998