National Insitute for Basic Biology

National Insitute for Basic Biology

|
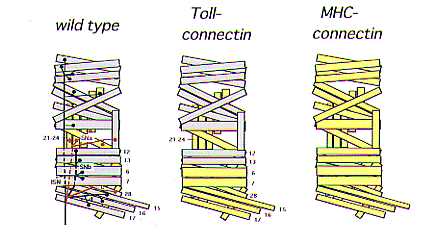
| Fig. 1. Ectopic expression of Connectin.
Schematic diagram showing wild type and ectopic Connectin expression on embryonic muscles and motor nerves. In wild type, Connectin is expressed on a subset of motoneurons (SNa and SNc, shown in red) and muscles (primarily lateral muscles, shown in yellow). In Toll-connectin, Connectin is ectopically expressed on a subset of ventral muscles (also shown in yellow). In MHC-connectin, Connectin is ectopically expressed on all muscles. |
|
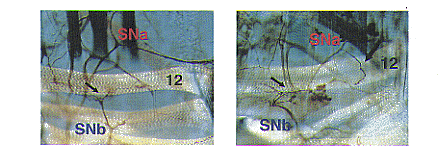
| Fig. 2. Connectin functions as an attractive guidance molecule.
In wild type, muscle 12 is innervated by SNb motoneurons (short arrow). Connectin-positive SNa motoneurons innervate more distal muscles. Ectopic Connectin expression on muscle 12 in MHC-connectin changed SNa trajectories: SNa sends an extra axon branch that forms an ectopic nerve ending on muscle 12 (large arrow). |