National Insitute for Basic Biology

National Insitute for Basic Biology

|
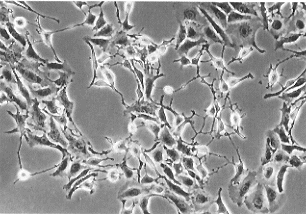
| Fig.1 Human retinal PECs showing a neuron-like morphology. Cells were isolated from the eye of an 80-year-old donor and their growth was enhanced by the EdFPH medium. Cells were then cultured in the standard medium for about30 days. |
|
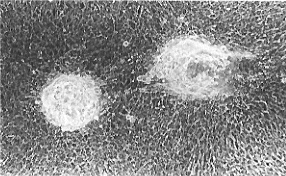
| Fig.2 Lentoids formed in the culture of the iris PECs of newly hatched chicks. Cell passaged in the EdFPH medium for several times were cultured with ascorbic acid for about 30 days. |