National Insitute for Basic Biology

National Insitute for Basic Biology

|
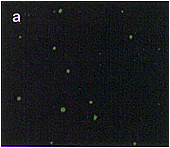
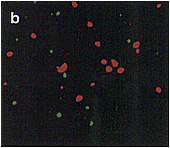
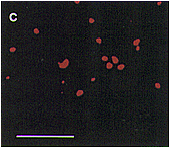
| Fig. 1. Localization of aconitase and isocitrate lyase within a cell from a pumpkin cotyledon, as analyzed by double immunofluorescence staining. Pumpkin seedlings were grown for 5 days in darkness. A thin section of a cotyledon was double-stained first with aconitase-specific antibodies which were visualized by use of FITC-conjugated second antibody, and then with isocitrate lyase-specific antibodies that were visualized by use of rhodamine-conjugated second antibody. (a) Immunofluorescent image due to FITC. (b) Immunofluorescent images in a and c are superimposed. (c) Immunofluorescent image due to rhodamine of the same field as in (a). Bar in panel c indicates 10Ám. Magnification in a, b and c is the same. |
|
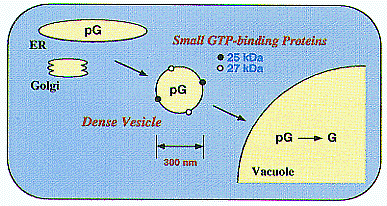
| Fig. 2. Dense vesicles mediate the final step in the delivery of seed proteins to vacuoles in developing pumpkin ( Cucurbita sp.) cotyledons. Two different, small GTP-binding proteins of 25 kDa and 27 kDa might function in the targeting and/or fusion of the vesicles to the vacuolar membranes or in the budding of the vesicles. |