2.6@ Crosses of mutants defective in the sporophyte formation
Takako Tanahashi
Introduction
Double
disruptants of PpLFY1*1
and PpLFY2*1 have
drastically decreased rates of sporophyte formation (less than 1%), compared to
that of the wild type (more than 90%). Most of the leaky sporophytes have
aberrant morphology and spores in those rarely germinate. By contrast,
gametophores of the double disruptants show no phenotypic difference from those
of the wild type. This is a cross method between the wild type and the PpLFY double disruptants utilizing the
self-sterile character of the latter. We obtained sporophytes originated from
cross-fertilization by wild-type sperm of egg cells of PpLFY disruptants. These are distinguished from other sporophytes
formed on the double disruptants by their morphology and the germination rate
of the spores they contain.
Our
method is especially effective in crosses of mutants defective in sporophyte
formation for the following two reasons.
1. The maternal
strain is easily identified when crossing two strains with no morphological
difference in their gametophores.@
2.
Jiffy-7 (medium used, see section 2.3) increases the efficiency of the
sporophyte formation.
@(*1 homologs of FLORICAULA/LEAFY genes in Physcomitrella
patens.@ Tanahashi et al. Development 132 1727-1736
(2005))
Procedures
1) Inoculate
the wild-type and the double disruptant mosses (protonemata or gamtophores) on
a Jiffy-7 pad (3 cm in diameter before expansion).
2) Grow
mosses under long day conditions (24L or 16L8D) at 25ºC
for 1-1.5 months and then under short day conditions (8L16D) at 15ºC for 3 weeks in separate plastic boxes (Fig.
1A).@ Antheridia and archegonia
differentiate on gametophore shoot apices.
3) Check
there are archegonia fully open and having uncolored neck canals (Fig. 2).
4) Place the wild-type and the disruptant mosses into a same
plastic box (Fig. 1B).
5)
Submerge these mosses with distilled water (Fig. 1C).@ Place it with gentle shaking for 30 seconds, and then remove the water
above the moss tissue by decanting (Fig. 1D).
6)
Continue the culture under short day conditions at 15ºC
for another 5 weeks.@ Collect
sporophytes formed on the double disruptant gametophores and examine the
genotypes of the progeny.
Result (all the
values are for colonies of PpLFY
double disruptants)
|
@No. Jiffy-7 used. |
@@@ 71 |
|
@No. gametophores with
archegonia@@@@@@@@@@@@@ |
@18019 |
|
@No. sporophytes* formed |
@@ 141 |
|
@No. sporophytes analyzed for
their genotype@@@@@@@@@@@@@@@@@@@ |
@@@ 87 |
|
@No. sporophytes formed by cross-fertilization@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@@ |
5
(normal morphology, @ germination rate was good) |
|
@No. sporophytes formed by
self-fertilization @@@@@@or parthenogenesis@@@@@@@@@@@@@@@@@@@@@@@@@@@@@ |
82
(largely abnormal morphology, @@@germination rate was bad) |
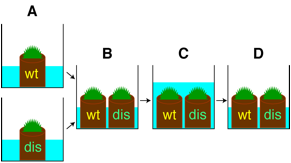
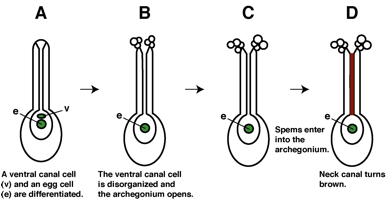
Fig. 1 Outline of crosses.
(wt; wild type, dis; PpLFY
double disruptant)
|
Fig. 1 Outline of crosses. (wt; wild type, dis; PpLFY
double disruptant) |
Comments
For
the present, the most effective way for the success of crossing is just;
1. Growing
healthy mosses
2. Performing
crosses with proper timing.
Therefore
you had better prepare MANY mosses to ensure success!
The
following might increase the efficiency of cross-fertilization, though we have
never tried this.
1. Repeating submergence several additional times. (If a
sporophyte is not formed, new archegonia will differentiate successively on a
shoot apex.)
2. Concentrating sperm by centrifugation and applying this close to an archegonium.