11.2 RNA
interference
Yuji
Hiwatashi
Introduction
RNA
interference (RNAi) takes advantage of the unique ability of double-stranded
RNA (dsRNA) molecules to induce posttranscriptional gene silencing in a highly
specific manner. An RNAi construct is introduced to protoplasts by PEG-mediated
transformation. RNAi becomes effective at the 2 to 3 cell stage after
protoplast regeneration. A loss-of-function
phenotype of FtsZ1, the larger chloroplast than in wild type, is observed only in
an apical cell at the three or more cell-stage of protonemal growth, 6 days
after transformation. RNAi for GFP is effective 4 days after transformation,
and GFP signals were diminished in all cells.
Steps for RNAi experiments:
1) Clone a
DNA fragment of a target gene into the entry vector pENTR/D-TOPO
2) Transfer
the DNA fragment in pENTR/D-TOPO to a destination vector pPI1 using the GATEWAY
system.
3) Co-transform
protoplasts with both the pPI1 derived-RNAi plasmid and a plasmid harboring a visual
expression marker.
4) Observe
regenerated protoplasts expressing the visual marker gene.
Materials
・ pENTR
Directional TOPO Cloning Kit (Invitrogen)
・ Wizard
Mini-Prep kit (Promega)
・ Hi
Speed Midi kit (QIAGEN)
・ Reagents
for PCR amplification
・ Reagents
for agarose gel electrophoresis
・ Gateway
LR Clonase Enzyme Mix (Invitrogen)
・ QIAquick
gel extraction kit (QIAGEN)
・ Reagents
for PEG-mediated transformation
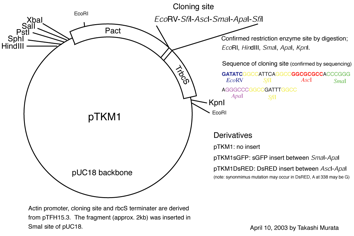
・ pPI
plasmid (see fig. 1). This gateway
plasmid for RNAi was originally designed and constructed by Dr. Tsuyoshi
Nakagawa (Shimane Univ., Japan) and modified by Y. Hiwatashi.
Procedure
1. Cloning of
a gene of interest into the entry vector pENTR/D-TOPO.
Refer to
the pENTR Directional TOPO Cloning Kit manual (cat.no.K2400-20).
1) Design
your PCR primer with CACC at the 5’ end (modification of the 3’ primer is not
necessary). Amplify your gene with a
proofreading enzyme (see below).
PCR total 20
µl
1
ul template cDNA
2
ul 10x KOD+ buffer
2
ul 2.5 mM dNTPs
0.8
ul 25 mM MgSO4
0.6
ul 10 pmol/ul primer1
0.6
ul 10 pmol/ul primer2
0.4
ul 1 u/ul KOD+
12.6
ul H2O
PCR cycle
94C,
2 min
94˚C,
15 s ━━┓
Tm-5˚C,
30 s x 30
68˚C, 1 m/1kb
━━┛
4˚C
2) Mix your
PCR product with the pENTR vector, transform E.coli, and plate.
TOPO reaction total 1.5 µl
0.25
ul Salt
0.5
ul H2O
0.25
ul pENTR/D-TOPO
0.5
ul PCR product (5-20 mg)
↓
Incubate
at room temperature for more than 5 min.
↓
Transform competent cells XL10-GOLD with all the solution,
then spread all the cells on an LB plate supplemented with 50 mg/l kanamycin,
and incubate at 37˚C overnight.
3) Select
positive clones by colony PCR.
4) Mini-prep
a positive plasmid with a Wizard Mini prep Kit or equivalent.
5) Digest the
plasmid with the appropriate restriction enzymes.
Restriction enzyme treatment total 10 µl
3
ul Plasmid
1
ul 10 x H
1
ul 0.1% BSA
0.5
ul 10 u/µl EcoRV
0.5
ul 10 u/µl NotI
4
ul H2O
↓
Incubate at 37˚C for 1 hrs.
↓
Perform gel-electrophoresis.
6) Confirm
the direction and sequence of the fragment by sequencing
2. Transfer
the DNA fragment cloned in pENTR/D-TOPO into the RNAi vector pPI1 (Figure 1).
Refer to the
Gateway LR Clonase Enzyme Mix manual (cat.no.11791-019).
1) Digest the
pPI1 vector with a restriction enzyme togenerate a linear form.
1.5
ul 1 µg/µl pPI1
1
ul 10 x H
0.5
ul 10 u/µl Xho I
8
ul H2O
↓
Incubate at 37˚C for more than 1 hrs.
↓
Perform gel-electrophoresis.
↓
Recover the digested plasmid with QIAquick Gel extraction
kit.
2) Perform the
LR reaction.
1
ul Entry clone (supercoiled, ~60 ng)
1
ul ~ 60 ng/µl linearized pPI1
0.8
ul 5x LR Clonase Rxn. Buffer
0.4
ul TE
↓
Add 0.8 µl of LR clonase enzyme mix, and then mix by
pipetting
↓
Incubate at 25˚C for more than 2 hrs.
↓
Add 0.4 µl of proteinase K, and then mix by
pipetting. Incubate at 37˚C for 10
min.
3) Transform
competent cells DH10B (or DH5alpha) with the product from the previous step. Spread all the cells on an LB+Amp plate and
incubate at 30˚C overnight.
4) Select a
correct RNAi vector by colony PCR. Since
the LR reaction allows the inverted direction of an intron in the RNAi vector,
confirm its direction by PCR. Confirm the
integration of the fragment, followed by the direction of the intron.
First colony PCR(total 20µl)
colony
inoculation
2
ul 10xEx Taq
2
ul 2.5 mM dNTPs
0.5
ul 10 pmol/ul 35S mini-F (CTAATCTTCGCAAGACCCTTCCTC)
0.5
ul 10 pmol/ul antisense primer
0.125
ul 5 u/ul Ex Taq
14.875
ul H2O
PCR cycle
94˚C,
30 s ━━┓
58˚C,
30 s x 30
72˚C, 1 m/1 kb ━━┛
4˚C
Second colony PCR (total 20 µl)
2
ul 10xEx Taq
2
ul 2.5 mM dNTPs
0.5
ul 10 pmol/ul 35S mini-F
0.5
ul 10 pmol/ul GFP1r1KpnI or GFPf1SpeI
0.125 ul 5 u/ul Ex
Tag
14.875 ul H2O
GPA1r1KpnI (ACCGGTACCTGCATATAACCTGC)
GPAf1SpeI (GATACTAGTGGTCGGTAACGGTCGG)
PCR cycle
94˚C,
30 s ━━┓
58˚C,
30 s x 25
72˚C, 1 m ━━┛
4˚C
The amount
of PCR amplified fragment with obtained using the 35S mini-F and GPA1r1KpnI
primers should be greater than that with 35S mini-F and GPAf1SpeI primers on
agarose gel electrophoresis in candidate clones.
5) Mini-prep
a positive clone with a Wizard Mini Prep Kit (Promega) or equivalent.
6) Digest
the plasmid with the appropriate restriction enzymes.
2
ul plasmid
1
ul 10 x M 10x M
0.5
ul 10 u/µl Xho I 10 u/µl XhoI
0.5
ul 10 u/µl Sac I 10
u/µl KpnI
6
ul H2O
↓
Incubate at 37˚C for more than 1 hrs.
↓
Perform gel-electrophoresis.
7) Sequence
the plasmid with the 35S mini-F and reverse primers.
8) Prepare
the plasmid on a large-scale with the QIAGEN hi Speed Midi Kit (QIAGEN).
3. Introduction
of RNAi vector to a protoplast.
Protoplasts
are co-transformed with the constructed RNAi plasmid and a visual selection
marker plasmid, since the pPI1 vector itself has no marker cassette. For a
visual marker plasmid, pTKM-GFP, pTKM-RFP, and pTKM-DsRED2 are used.
1) Prepare a marker
plasmid.
2) Co-transform
protoplasts with a mixture of 10 µg of the RNAi plasmid and 10 µg
of a marker plasmid. As a control, co-transform with the original pPI1 plasmid
instead of the RNAi plasmid.
3) Incubate
the transformed protoplasts.
4. Observation
Regenerated
protonemata expressing a marker gene such as GFP and mRFP are observed.
Figure 1. pPI1
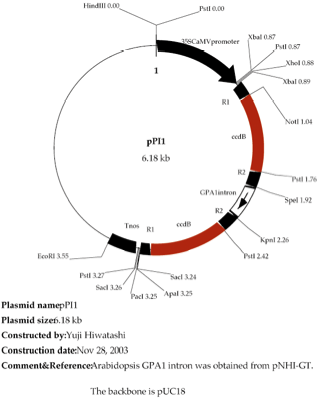
Figure
2. pTKM-GFP