
 |
LABORATORY OF NEUROCHEMISTRY |
| Associate Professor: | SASAOKA, Toshikuni |
|
Our major research interest is to understand a physiological role of dopaminergic system in animal behavior, especially eating behavior using genetically altered mice, both transgenic and gene knockout mice. In addition we develop a novel method of conditional mutagenesis in mice in order to analyze the function of the gene of interest in detail. We analyze function of neurotransmitter receptor complex as a whole by using biochemical methods of analysis of the dystrophin complex on the skeletal muscle membrane. |
I. Role of dopaminergic transmission in eating behavior |
|
The dopaminergic system is implicated in the regulation of the several peptide hormones in the pituitary, the modulation of locomotor activity, the modulation of synaptic plasticity and the neural development. The dopaminergic system is also implicated in control of emotion, motivation and cognition. Dysfunction of dopaminergic system can result in several neurological and psychiatric disorders such as Parkinson’s disease and schizophrenia. |
 |
|
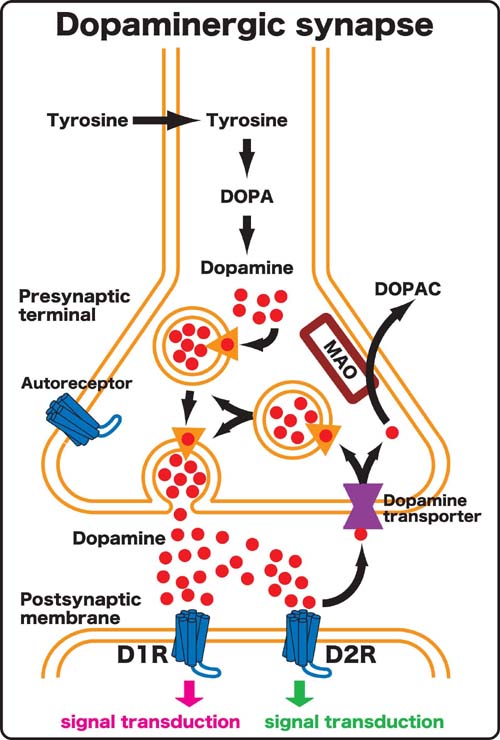
Figure 1. Schematic drawing of dopaminergic synapse. |
|
In mammals five subtypes of dopamine receptor (D1R, D2R, D3R, D4R and D5R) are identified and divided into two subgroups referred to as D1-like (D1R, D5R) and D2-like (D2R, D3R and D4R) receptors on the basis of their gene structure and their pharmacological and transductional properties. D1R and D2R are most abundantly and widely expressed in the brain and often play a role synergistically. D1R has an opposite property to D2R with respect to the intracellular signal transduction. In order to understand a role of dopaminergic transmission in behavior of interest, it is necessary to delete both D1-like and D2-like receptors. We have generated D1R/D2R double knockout (DKO) mice by crossing between D1R knockout (KO) and D2R KO mice, and observed that D1R/D2R DKO mice exhibited impairment in eating behavior and premature death. To investigate molecular mechanism of eating we have generated mutant mice in which the expression of dopamine receptor could be controlled temporally by tetracycline system on the D1R/D2R DKO background. We plan to establish and analyze the mutant mice. |
II. Developing a novel conditional mutagenesis method in mice. |
 |
|
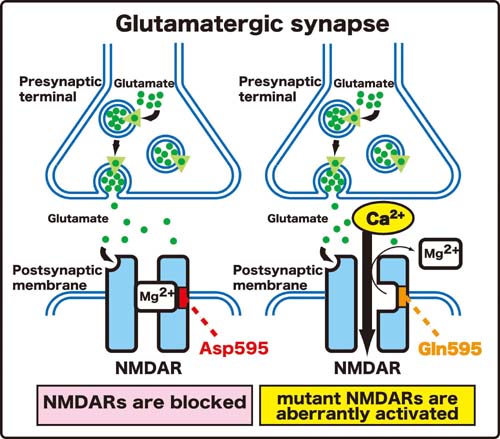
Figure 2. A single amino acid substitution (595th asparagine to 595th glutamine) of NR2A subunit leads to an aberrant activation of NMDAR. |
|
The aim of the study is to overcome the limitations of the conventional mouse molecular genetic approach in the functional analysis of target genes. We substituted one critical amino acid residue of N-methyl-D-aspartate receptor (NMDAR), leading to NMDAR activation. The NMDARs are widely expressed in the nervous system, fundamental to excitatory neurotransmission, and play a number of important roles at different brain loci and time points. The NMDARs act as a coincidence detector and are not only important for neuronal differentiation, migration, and survival but are also critical for activity dependent synapse formation. It is suggested that the aberrant activation of NMDAR causes excitotoxicity, leading to neuronal death in various neurological diseases. That the Ca2+ permeability through NMDAR is blocked by magnesium (Mg2+) in a voltage-dependent manner indicates an essential role of NMDAR as a coincidence detector. Functional NMDARs consist of NMDAR1 (NR1) subunit and at least one subunit of NMDAR2A-2D (NR2A-NR2D). The NR1 subunit is ubiquitously expressed in the brain, while NR2 subunits have a more specific spatial distribution. It has been shown that the NR1/NR2A complex expressed in cultured cell is highly sensitive to the voltage-dependent Mg2+ block and that the substitution of asparagine (Asp595) by glutamine (Gln595) in the second transmembrane domain of the NR2A subunit results in a reduction of the Mg2+ block of the NR1/NR2A complex (Figure 2). |
 |
|
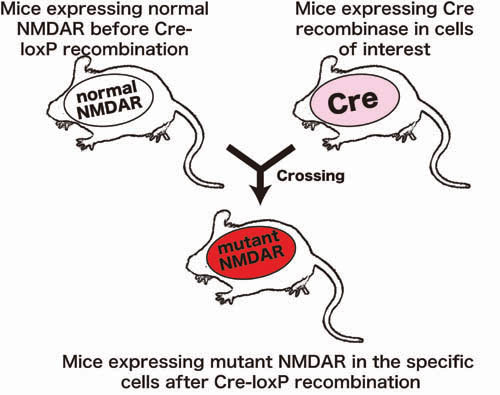
Figure 3. Conditional mutagenesis in mice. First, mutant mice expressing normal NMDAR molecule before Cre-loxP recombination were generated. Second, transgenic mice expressing Cre recombinase in cells of interest were generated. Third, these two mouse lines were crossed to generated mice expressing mutant NMDAR molecule in the cells in which Cre-loxP recombination was executed. |
 |
|
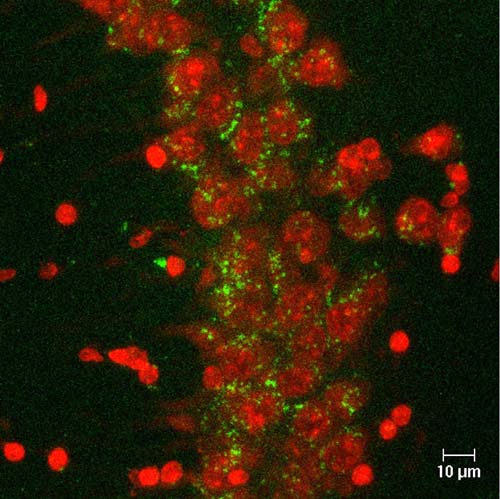
Figure 4. Detection of cells in which recombination was executed by Cre-loxP system by expression of green fluorescent protein (green) in the hippocampus. Neurons were stained by NeuroTrace (red). |
|
However, the role of Asp595 of the NR2A subunit and the effects of substitution of it with Gln595 on the function of NMDAR in vivo remain to be clarified. We develop conditional mutagenesis method in mice using Cre-loxP recombination (Figure 3 and 4). By our method, we accomplished conditional substitution of the amino acid in mice and our mutant mice exhibited aberrant NMDAR activation and a neurological phenotype, similar to that of mouse models of neurological disorders. The development of our mutant mice should contribute to understanding the function of the critical amino acid residue and the molecular mechanism of neurological disorders. Our new method is vastly applicable to functional analysis of any desired gene and should contribute to studies on the structural and functional relationships of relevant genes. |
III. The molecular architecture and the physiological role of the sarcoglycan complex (SGC) |
|
Sarcoglycans (SG) are trans-sarcolemmal glycoproteins, which associate together to form SGC and present in the sarcolemma. SGC, together with dystrophin and the dystroglycan complex comprises the dystrophin complex, which is considered as the mechanical link between the basement membrane and the intracellular cytoskeleton for protecting the sarcolemma from mechanical stress during muscle contraction. Each of four SG subunits (α-, β-, γ- and δ-SG) is responsible for four respective forms of SG-deficient muscular dystrophy, sarcoglycanopathy (SGP). All of the SGs and sarcospan are absent in the sarcolemma in any form of SGP, suggesting that the SGC is not assembled if a single subunit of the SGC is absent. To understand a physiological role of the SGC, we have generated the β-SG KO and γ-SG KO mice. The β-SG KO and γ-SG KO mice developed progressive muscular dystrophy and all SGs and sarcospan were absent in the sarcolemma of the β-SG KO and γ-SG KO mice. The dystrophin complex isolated from the skeletal muscles of β-SG KO mice was biochemically unstable in the absence of the SGC and sarcospan. This indicates that SGC and sarcospan play an important role in stabilizing the dystrophin axis connecting the basement membrane and the cytoskeleton. We plan to analyze a molecular architecture and a physiological role of dopamine receptor complex as a whole by applying analytical method of the SGC. |
Publication |
|
Mizuno, Y., Guyon, J. R., Watkins, S. C., Mizushima, K., Sasaoka, T., Imamura, M., Kunkel, L. M., Okamoto, K. (2004) Beta-Synemin localizes to regions of high stress in human skeletal myofibers. Muscle and Nerve 30, 337-346 |
